The therapeutic landscape in the field of atopic dermatitis is changing. The spectrum of biologics and JAK inhibitors has expanded. Study data attest to the high efficacy of all currently available modern systemic therapeutics. The most significant differences relate to the respective side effect profiles and handling.
According to the current understanding of the disease, atopic dermatitis is a systemic disease with a multifactorial etiology that includes epidermal and immunological components, explained Peter Radny, MD, dermatologist in private practice and director of the Derma-Study Center in Friedrichshafen, Germany [1]. Even though there are very different courses of the disease, neurodermatitis sufferers have one thing in common: “The itching is very stressful for the patients,” emphasizes the dermatologist [1].
Patient education: communicating the explanatory model
Often, as a consequence of the itching, sleep disturbances occur, resulting in concentration difficulties and reduced performance during the day. The itch-scratch cycle and accompanying scratch lesions also lead to irritants penetrating the skin more easily. In addition to symptoms such as xerosis and eczema, a secondary bacterial or mycotic infection may develop. Communicating to patients that atopic dermatitis can be explained by an interaction of genetic predisposition, triggering and triggering factors can prove to be a difficult undertaking in some cases. “You have to meet patients where they are,” summarizes the dermatologist [1]. This also means addressing patients’ subjective explanatory models. Patience and understanding are factors that promote adherence. That dysregulation of the immune system is involved in atopic dermatitis is shown, for example, by the fact that increased levels of proinflammatory cytokines have been demonstrated in both lesional and non-lesional skin of atopic dermatitis patients [2].
Anti-inflammatory therapy: regular assessment of progression.
The step therapy regimen embodied in the guideline is still valid, the speaker said, although the guideline is continually revised to incorporate newly approved agents [1,3]. While mild eczema can usually be controlled by anti-inflammatory local therapy (topical cortisone preparations or calcineurin inhibitors), systemic therapy should be considered at the latest at stage 3 or 4 severity [1]. In particular, insufficiently controlled moderate or severe atopic dermatitis can significantly impair the quality of life of those affected. Anti-inflammatory systemic therapy can interrupt the characteristic inflammatory cascade, often resulting in significant symptom relief. In order to assess the course of therapy in acute phases or in the stabilization and maintenance phase under systemic treatment, the lecturer recommends the use of the “Neurodermatitis Evaluation Test Tool” (NETT), a meaningful and practicable questionnaire [4]. “This takes only 1-2 minutes and documents that everything has been considered under therapy,” the speaker elaborates [1]. Among other things, the severity, and the effects of the therapy after a certain treatment period (e.g. after 4-6 weeks) are recorded.
Biologics target extracellular targets
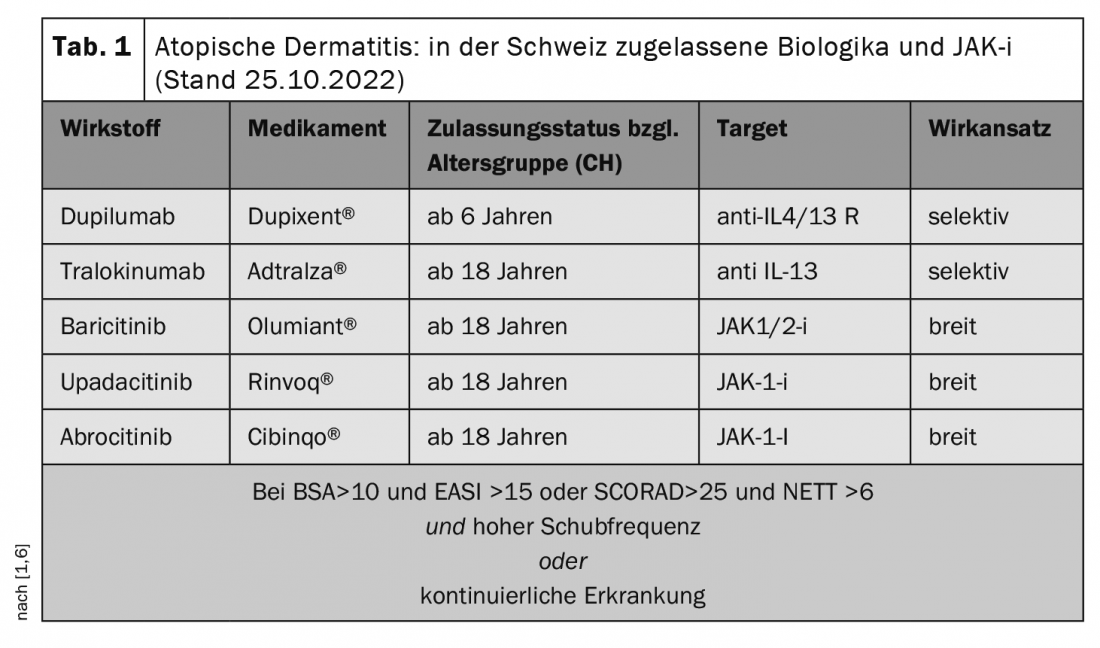
Effective and well-tolerated systemic therapy can significantly improve patients’ functional capacity and well-being. “Dupilumab was the first antibody to be approved and the one we have worked with in this indication the longest to date,” said Dr. Radny [1]. Accordingly, the most data and experience are currently available on this biologic, which targets the alpha chain of the IL-4 type I receptor and the IL4/IL13 type II receptor, thus blocking the action of two key cytokines of atopic inflammation [3]. Dupilumab has been approved for the indication neurodermatitis in Germany for adults since 2017 and for adolescents aged 12 years and older since 2019. This was followed in 2021 by the approval of the anti-IL-13 antibody tralokinumab. Both biologics are administered as injections. TB screening is not required and vaccination with inactivated or inactivated vaccines is possible. The most common side effects of the two biologics are conjunctivitis and blepharitis, although these occur comparatively less frequently with tralokinumab. Patients who show a good response but have conjunctivitis that persists despite lid margin care, eye drops, and hyaluronic tear substitute film, the speaker recommends considering ciclosporin A 1% as an interval treatment. The prerequisite is that patients are motivated and compliant. “Ciclosporin eye drops can be a really good solution,” emphasizes the dermatologist [1].
JAK inhibitors interfere with intracellular signaling pathway
Janus kinase inhibitors (JAK-i) have a slightly different mechanism of action than biologics. This is because they induce their anti-inflammatory effects by affecting the intracellular JAK-STAT pathway. The rapid onset of action of this class of compounds may be beneficial for patients in certain situations, such as those with an acute relapse who have an important event imminent [1]. The use of a JAK-i may also be preferable in patients who prefer oral therapy to injection treatment. However, more laboratory tests are required before and during therapy with JAK-i than with biologics. Mandatory infection prophylaxis includes TB exclusion diagnosis and checking hepatitis vaccination status. Possible side effects of JAK-i include nasopharyngitis, upper respiratory tract infections, headache, and herpes simplex. Conjunctivitis and blepharitis, on the other hand, are rare [5].

Systemic agents in the pipeline – “coming soon”?
The currently approved biologics and JAK-i all have very good response rates. Dr. Radny [1] emphasized that the primary factor in deciding which systemic therapy agent to use should be what is most appropriate for the individual patient. In reality, however, there are patients who do not achieve satisfactory treatment results, even though every effort is made. The treatment arsenal could soon be expanded to include additional systemic therapeutics. In addition to the anti-IL-13 antibody lebrikizumab, the monoclonal antibody nemolizumab directed against IL-31 is also in advanced stages of clinical development. And in JAK-i, two topically applied agents are in phase III clinical trials: data on both ruxolitinib (anti-JAK-1/2) and delgocitinib (anti-JAK-1/2) are promising. Furthermore, we look forward to further study results on etrasimod, an S1P receptor modulator, for which data from a phase IIa study are currently available [1].
Congress: Bodensee Dermaconsil
Literature:
- “Current developments in atopic dermatitis,” Peter Radny, MD, Lake Constance Dermaconsil, Oct. 22, 2022.
- Brunner PM, Guttman-Yassky E, Leung DY: The immunology of atopic dermatitis and its reversibility with broad-spectrum and targeted therapies. J Allergy Clin Immunol 2017; 139(4S): S65-S76.
- Update system therapy for atopic dermatitis to the guideline atopic dermatitis, development stage: S2k – 2020. AWMF Registry Number: 013-027. www.awmf.org,(last accessed 10/25/2022).
- Kurzen H, et al: Atopic dermatitis – A practical treatment pathway. www.prof-kurzen.de/upload/59487095-Kurzen-et-al.-AD-Behandlungspfad-2020…,(last accessed 25 Oct 2020).
- Simpson EL, et al: Baricitinib in patients with moderate-to-severe atopic dermatitis and inadequate response to topical corticosteroids: results from two randomized monotherapy phase III trials. Br J Dermatol 2020; 183(2): 242-255.
- Drug Information, www.swissmedicinfo.ch,(last accessed Oct. 25, 2022).