Case report: In 2011, a 50-year-old patient was diagnosed with a cavernoma of almost 2 cm in the area of the pons, after passive mild sensory disturbances of the right side of the face. Progress controls showed stable conditions. In June 2015, sensory disturbances occurred again, which were almost completely regressed after one week. MRI showed the cavernoma to be slightly progressive in size.
Another week later, there was high-grade hyesthesia of almost the entire right side of the face, and shortly thereafter, right facial nerve palsy, vestibular disorders, vertigo, vomiting, and mild left hemiparesis. MRI showed a marked increase in size of the cavernoma in the setting of hemorrhage (Fig. 1) .
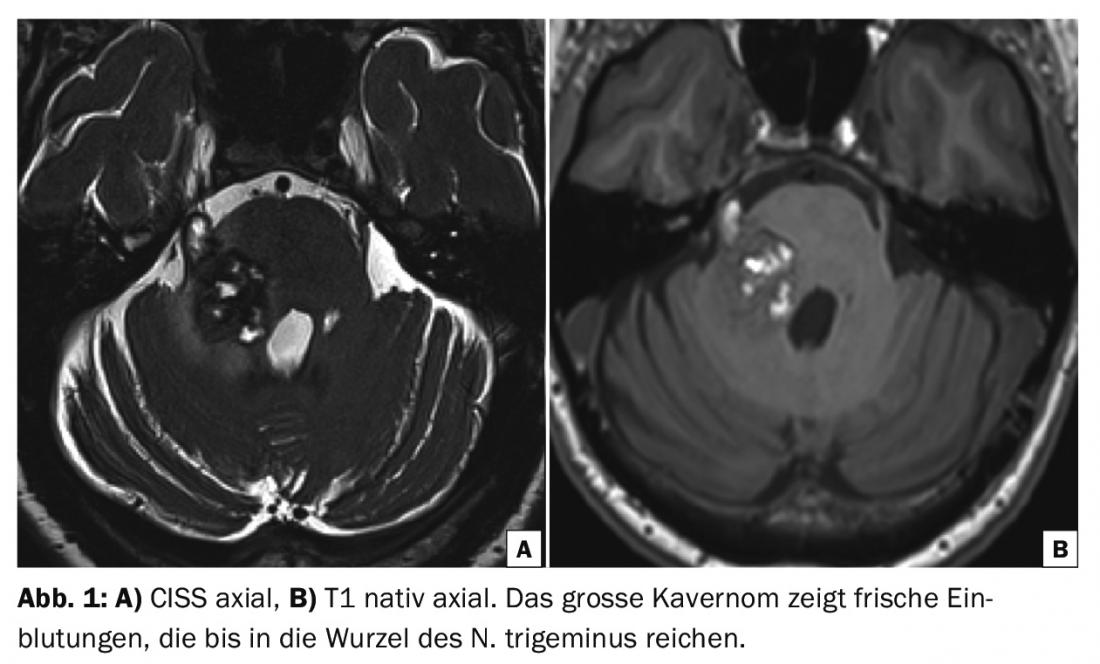
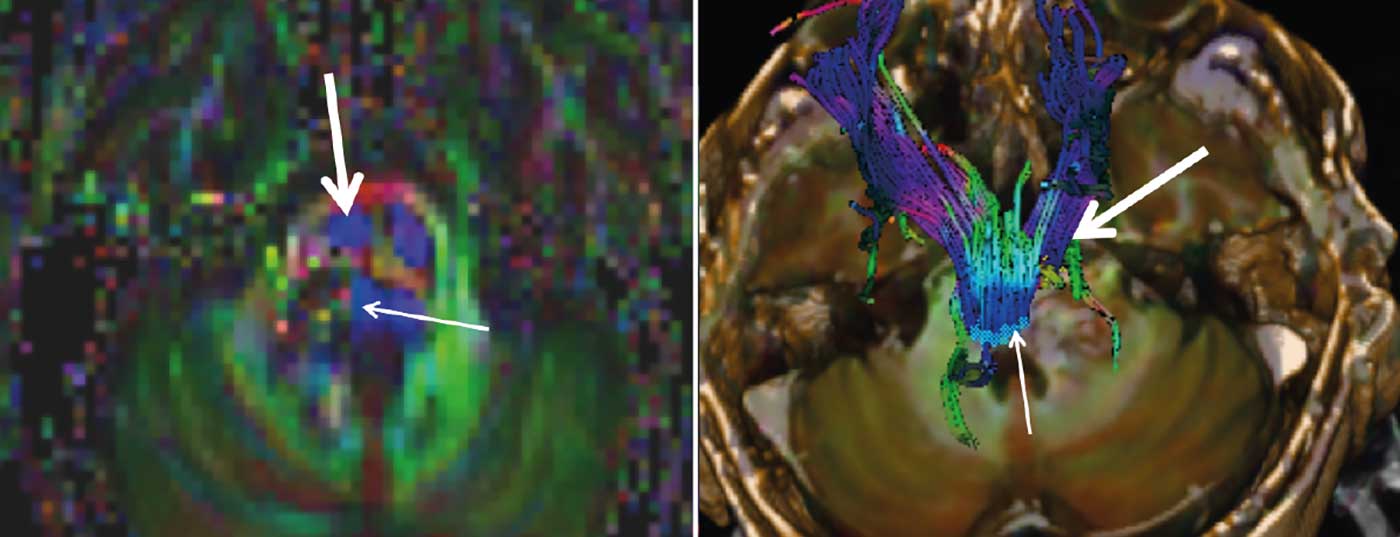
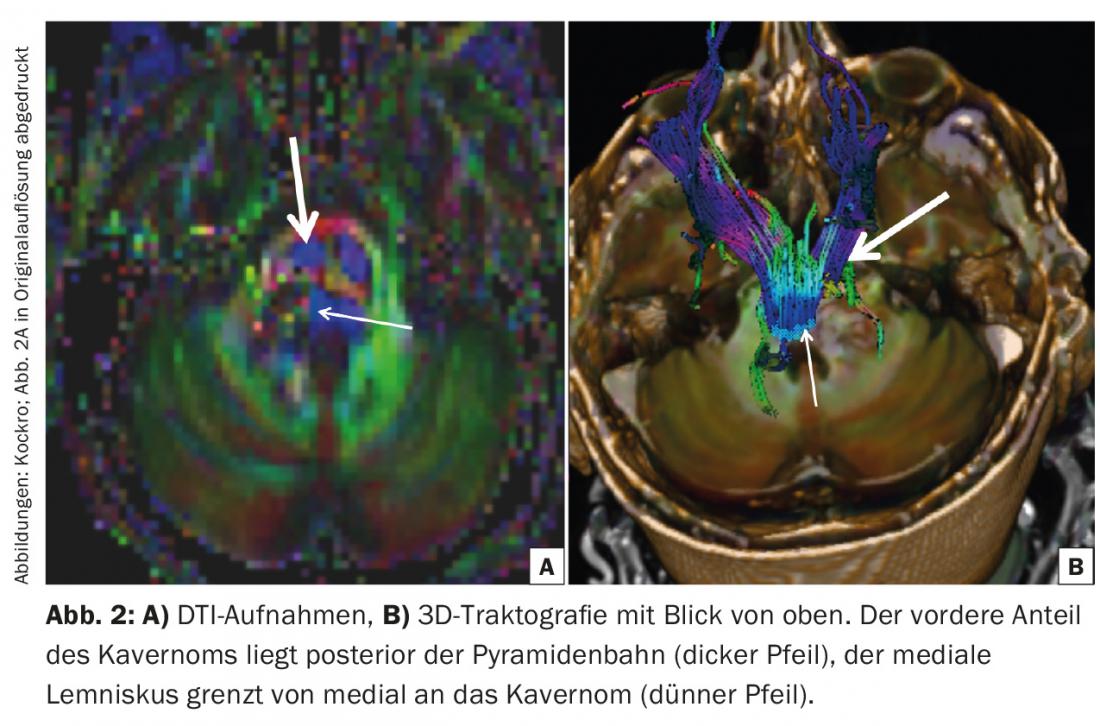
The indication for neurosurgical intervention was given. Preoperative DTI tractography showed the right and left corticospinal tracts anterior to the cavernoma. The medial lemniscus (Fig. 2) was displaced medially. The cavernoma was prominent in the area of the right pons between the entrance area of the right trigeminal nerve and the core area of the facial/vesibulocochlear nerve. A fresh hemorrhage extended into the proximal bulb of the trigeminal nerve.
Surgical access was selected via an entry into the brainstem in the brachium pontis region approximately 8 mm posterior and 4 mm inferior to the entry site of the right trigeminal nerve. At this point, the hemorrhage of the cavernoma almost reached the surface of the brain stem. The craniotomy was located right retrosigmoidally at the angle of the transverse and sigmoid sinuses. Intraoperatively, the right horizontal cerebellar fissure was first dissected open to expose the target area at the brachium pontis. A horizontal incision parallel to the fibrous course of the brachium was used to aspirate the bleeding and then reach the deeper cavernoma. With almost no traction on the brainstem, the entire cavernoma could be detached from the brainstem and removed with the aid of the endoscope.
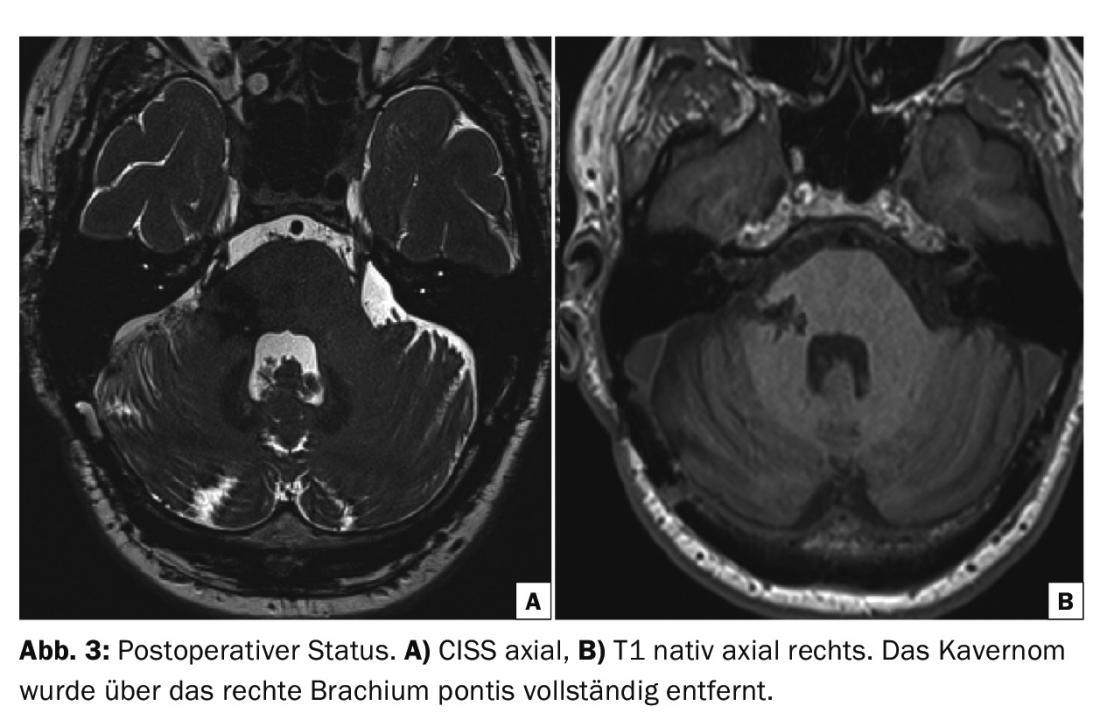
The postoperative course was unremarkable and the patient was discharged to a neurological rehabilitation clinic on the ninth postoperative day. Three months after surgery, apart from mild partial trigeminal hypesthesia, there were no neurological deficits and the patient had already returned to work. MRI showed the cavernoma completely removed and the brainstem was unremarkable (Fig. 3).
Discussion: Cavernomas are vascular malformations consisting of thin-walled venous caverns contiguous directly or separated by thin connective tissue. Direct arterial inflow is not found; rather, carvernomas are hemodynamically characterized by slow venous blood flow with partial intraluminal thrombosis. Intra- or extralesional hemorrhage of various ages contributes to a diverse cavernoma morphology, and resorption processes often result in hemosiderin deposition inside and outside the lesion. Often, a dilated vein (DVA, “deep venous anomaly”) occurs in association with the lesion. The size of cavernomas can vary from a few millimeters to several centimeters, and intraoperatively the typical mulberry-like appearance of lobulated dark red caverns is seen.
Since the era of MRI, cerebral cavernomas have been diagnosed with increasing frequency. Their prevalence is estimated to be 0.4-0.5% of the population [1] and they occur both sporadically and occasionally in families. On MRI, cavernomas appear as well-defined roundish structures, which on T2-weighted images often have a central area of mixed signal intensity, corresponding to hemorrhages of different ages, surrounded by a ring of decreased signal intensity, corresponding to hemosiderin deposits. On native CT, cavernomas often show nodular heterogeneous hyperintensity with variable weak contrast enhancement. Occasionally, slight punctate or scaly calcifications are seen. In acute bleeding, it is often not possible to secure a cavernoma as the cause of bleeding on CT.
The clinical picture of cerebral cavernomas is determined on the one hand by the localization and on the other hand by hemorrhages. The spectrum therefore ranges from asymptomatic course to epileptic seizures or severe focal deficits following hemorrhage in eloquent areas.
Cavernomas in the brainstem are often characterized by rapid onset of neurologic symptoms due to their location in the midst of closely contiguous neuronal pathways and nuclei. If there are multiple episodes of minor hemorrhage within the lesion, the cavernomas enlarge in a balloon-like fashion, and surrounding structures are displaced and compressed. Pressure on intrinsic brainstem vessels thereby increases the risk of regional inferior perfusion. The bleeding probability of cavernomas has been widely studied. For supratentorial cavernomas, data range from 0.25 to 2.6% per year [1–3], cavernomas of the brainstem show an increased risk with up to 7% annual bleeding probability in symptomatic cavernomas [4].
Therapeutic management of brainstem cavernomas is influenced by the balancing of several factors. In general, incidentally diagnosed asymptomatic cavernomas do not require neurosurgical intervention and are observed with MRI. On the other hand, any cavernoma that has bled and become symptomatic represents a risk, and given the statistically increased likelihood of further bleeding, microsurgical extirpation should be considered as a therapeutic option. This is especially true in cases of recurrent bleeding and progressive neurologic deterioration. The positional relationship of the cavernoma to the brainstem surface and to the position of the cranial nerve nuclei and neuronal pathways is directly related to surgical accessibility and the individual risk profile of surgery. Therefore, these facts also play a role in weighing a surgical indication.
The success of an operation depends crucially on the preparation. In addition to anatomical understanding of the architecture of the brainstem and its surrounding structures, it is primarily MRI imaging that specifies the planning of a surgical corridor [5]. Although imaging is difficult given the tissue compression caused by space-occupying lesions and swelling, diffusion tensor imaging (DTI) sequences and tractography based on these sequences are usually successful in visualizing the corticospinal tract and the medial lemniscus. High-resolution techniques allow visualization of the core areas of the cranial nerves. With this information, the best possible surgical approach to the cavernoma can be defined, and planning begins by determining the possible least-risk pathways outward from the cavernoma and then adapting the proximal corridor. The entry zone into the brainstem takes into account in particular the course of the exiting cranial nerves and their core areas, the vessels at the brainstem surface, and finally the morphology of the cavernoma itself – always with a view to microsurgical accessibility of all cavernoma spaces without collateral damage. The goal is careful dissection along the surface of the cavernoma and its complete removal.
Literature:
- Engelmann R, et al: Epidemiology and natural history of cavernous malformations. In: Rigamonti D (ed.): Cavernous Malformations of the Nervous System. Cambridge University Press 2011; 9-14.
- Del Curling O, et al: An analysis oft he natural history of cavernous angiomas. J Neurosurgery 1991; 75: 702-708.
- Kndziolka D, Lunsford LD, Kestle JR: The natural history of cerebral cavernous malformations. J Neurosurgery 1995; 83: 820-824.
- Mathiesen T, et al: Deep and brainstem cavernomas: a consecutive 8-years series. J Neurosurgery 2003; 99: 31-37.
- Bertalanffy H, et al: Resection of cavernous malformations of the brainstem. In: Rigamonti D (ed.): Cavernous Malformations of the Nervous System. Cambridge University Press 2011; 143-160.
InFo NEUROLOGY & PSYCHIATRY 2016; 14(2): 34-35.