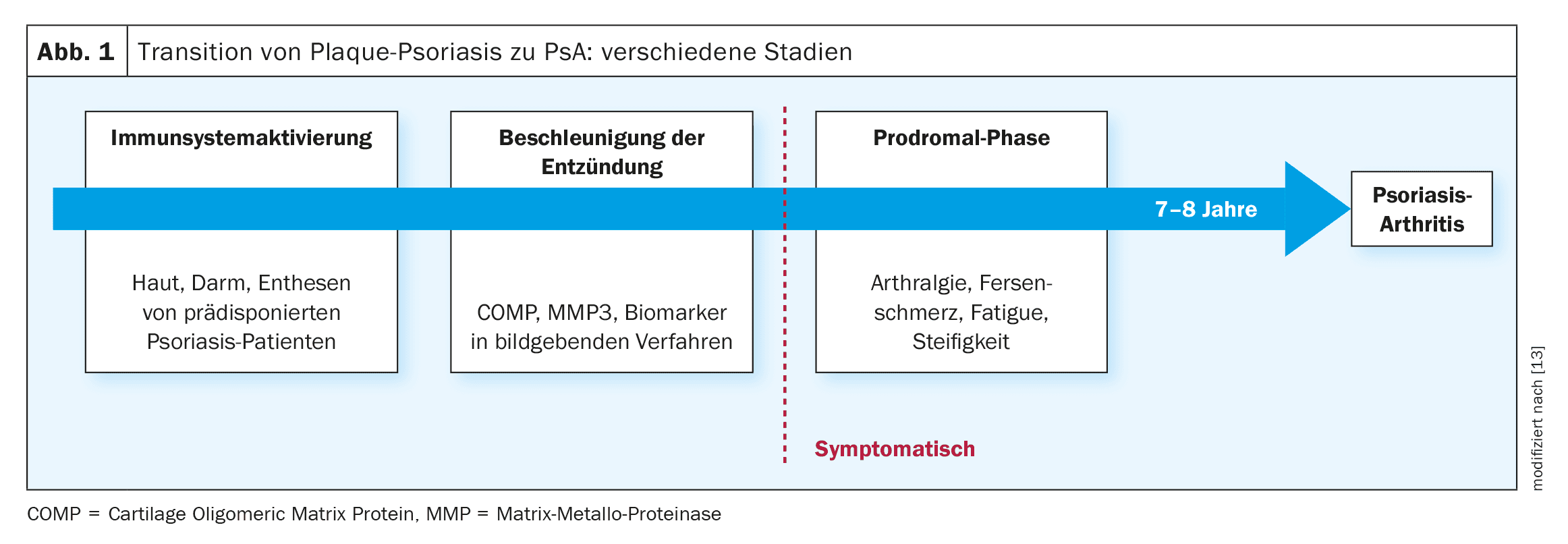
Psoriasis often affects the joints during the course of the disease – around a third of those affected develop psoriatic arthritis (PsA) over time. Improving early detection and developing preventive therapeutic approaches are the focus of various ongoing research projects. These include, for example, biomarker studies using omics technologies and intervention studies on the time-critical use of various biologics.
The key question is how the risk of psoriatic arthritis (PsA) can be predicted with certainty and whether PsA can be prevented by early adequate intervention, explained Professor Laura Coates, Nuffield Department of Orthopaedics, Rheumatology and Musculoskeletal Sciences, University of Oxford (UK) [1]. There is as yet no conclusive answer to this question, but numerous research initiatives are gradually shedding light on the subject. It has been known for some time that around 80% of patients have plaque psoriasis before they develop PsA, meaning that the main risk group is a defined patient population that can be identified via register data and medical records [2].
Predictors for PsA: risk models and self-monitoring approaches
Although there are currently no reliable methods or tests that can quickly, easily and reliably indicate the development of PsA and its later course, several research projects are looking into this topic. Researchers in Canada have developed a risk model that can be used to predict whether a psoriasis patient has a high risk of developing PsA within a period of 1 year or 5 years based on various patient characteristics [1,3]. And at this year’s EULAR, British scientists presented the results of a large-scale secondary analysis based on data from the UK CPRD (UK Clinical Practice Research Datalink), in which demographic risk factors for a higher probability of developing PsA were identified [4]. To this end, the researchers analyzed case data from 122,330 patients with plaque psoriasis. This showed that PsA manifested itself in 2460 of those affected within a median period of 5.7 years. The following demographic risk factors were identified: Age group 30-50 years, male gender, BMI >25.
Prof. Coates cited the iPROLEPSIS project and the PSORCAST study [1] as examples of digital health approaches in the area of risk assessment and early diagnosis of PsA. The iPROLEPSIS project is about implementing a personalized digital application that supports patients in disease management in a playful way and contributes to data collection in a real-world setting with the aim of gaining new insights into important factors that promote the development of PsA in patients with existing psoriasis [5]. The PSORCAST study aims to develop and validate a smartphone app that integrates digital, clinical and laboratory data to identify personalized PsA risk factors and facilitate early PsA diagnosis [6]. Patients can use this app for self-monitoring in everyday life.
Can PsA-specific biomarkers be identified?
The HIPPOCRATES consortium includes partner institutions from Belgium, Denmark, Germany, Ireland, Italy, Spain, Sweden, Switzerland, the Netherlands, the UK and the USA [7]. The aim is to test newly discovered markers and procedures that could be used for the early diagnosis of PsA in international patient cohorts. In addition, the HIPPOCRATES project is developing personalized treatment strategies to improve treatment response and inhibit the development of severe psoriatic arthritis. One basic idea is to enable early diagnosis and treatment by identifying molecular PsA biomarkers among patients with plaque psoriasis so that the effects of the disease can be mitigated. To this end, researchers are aiming to identify a molecular signature using multi-omics technology. In this context, a pilot study was carried out as part of the HIPPOCRATES project, reported the speaker [1]. Serum samples from 90 people taking part in a prospective psoriasis study (BioCOM) at St. Vincent’s University Hospital in Dublin (Ireland) were analyzed using quantitative proteomics (LC-MS/MS analysis), targeted affinity proteomics, metabolomics, lipidomics and genomics [1,8]. Of the 90 participants, 30 had established PsA according to CASPAR criteria, 30 had psoriasis without clinical musculoskeletal signs and 30 had psoriasis with clinical symptoms suggestive of early musculoskeletal involvement. The researchers were able to identify molecular markers that make it possible to differentiate between psoriasis and PsA. In ongoing studies, the multi-omics data is being integrated and analyzed using machine learning and AI.
| PsA risk factors |
| – Positive family history |
| – Higher psoriasis severity index (PASI) |
| – Longer duration of the psoriatic disease |
| – Arthralgia, stiffness Fatigue |
| – Overweight (BMI >35) |
| – History of uveitis |
| – History of thyroid disease |
| – Specific psoriasis localization (nail, scalp, gluteal) |
| – Hyperlipidemia |
| – Depression |
| – Smoking |
| – Trauma |
| to [1,12] |
“Windows of opportunity” for intervention with biologics?
In this context, drug interventions with biologics are based on the hypothesis that certain active substances can influence both skin symptoms and joint involvement through anti-inflammatory effects in such a way that progression is halted. It is assumed that the biologics are used in a time-critical manner.
- IVEPSA (“Interception in Very Early PsA study”) is an open prospective exploratory study that investigated whether early intervention with the interleukin (IL)-17A inhibitor secukinumab leads to a comprehensive remission of skin symptoms, pain and subclinical inflammation [9]. Inclusion criteria were nail or scalp infestation or a PASI (Psoriasis Area Severity Index) >6, as well as inflammatory or erosive changes on MRI or CT. The patients received treatment with secukinumab for 24 weeks. Clinical assessments of skin and joint disease were performed at baseline and after 12 and 24 weeks. 20 patients were included, 85% of whom reported arthralgia and 40% had painful joints on examination. 83% had at least one inflammatory lesion on MRI, most of them synovitis/enthesitis. Skin disease (PASI: p<0.002; BSA: p<0.003) and arthralgia (VAS pain: p<0.003) improved significantly after 24 weeks. The psoriatic arthritis magnetic resonance imaging scoring system ( PSAMRIS) and the synovitis subscore also improved significantly (p=0.005 and p=0.008 respectively). Erosions and enthesiophytes did not increase, while bone mass in the distal radius increased significantly (p=0.020) after 24 weeks.
- PAMPA (“Preventing Arthritis in a Multicenter Psoriasis At-risk Cohort”) is an ongoing randomized controlled double-blind multicenter study to evaluate whether treatment with the IL-23 inhibitor guselkumab can counteract the progression of subclinical structural joint-related abnormalities [10,11]. Patients with plaque psoriasis who have a high risk of PsA are included. The co-primary endpoint is the change in subclinical structural joint-related abnormalities (measured by power Doppler ultrasonography) from baseline to week 24 in the guselkumab group vs. the control group. Participants in the control group will not receive biologic therapy.
Both biologics mentioned – secukinumab (Cosentyx®) and guselkumab (Tremfya®) – are approved in Switzerland for the indication psoriatic arthritis, among others [14].
Conclusion
In summary, patients at high risk of PsA are a relatively easily identifiable population. Numerous research projects worldwide are focusing on the early detection and intervention of PsA. Although certain factors have now been identified that are associated with an increased risk of PsA in existing plaque psoriasis, there is a need to evaluate individual PsA risk factors more accurately. With regard to therapeutic interventions, the current data suggests that the use of biologics is highly likely to reduce the risk of PsA, although the appropriate timing for starting therapy and the mechanisms involved have not yet been fully clarified. In general, questions regarding patient acceptance and the ethics of long-term studies arise in relation to PsA prevention as a research topic. The willingness of psoriasis patients to participate in PsA prevention studies depends on various factors, according to Prof. Coates [1]. One advantage is that many of the active substances being investigated with regard to preventive effects on PsA are also effective against plaque psoriasis. However, the question of how a control arm can be operationalized in longer-term randomized studies so that it is ethically justifiable is a major hurdle for long-term study projects, according to the speaker [1].
Congress: World Psoriasis & Psoriatic Arthritis Conference
Literature:
- “Can we prevent psoriatic arthritis?”, Keynote lecture, Professor Laura Coates, IFPA Conference, 7th World Psoriasis & Psoriatic Arthritis Conference, June 27-29, 2024, Stockholm.
- Gottlieb AB, et al: Use of etanercept for psoriatic arthritis in the dermatology clinic: the Experience Diagnosing, Understanding Care, and Treatment with Etanercept (EDUCATE) study. J Dermatolog Treat 2006;17(6): 343-352.
- Eder L, et al: POS0019 Prediction of Psoriatic Arthritis Tool (PRESTO): Development And Performance Of A New Scoring System For Psoriatic Arthritis Risk. Annals of the Rheumatic Diseases 2023; 82: 215.
- Rudge A, et al. POS0964 Dynamic prediction of Psoriatic Arthritis in a cohort of Psoriasis Patients using UK Primary Care Electronic Health Records. Annals of the Rheumatic Diseases 2024; 83: 699-700.
- “iPROLEPSIS”, www.iprolepsis.eu,(last accessed 25.09.2024).
- “PSORCAST Study”, www.med.upenn.edu/inflammatory-arthritis/psorcast-study.html,(last accessed 25.09.2024).
- “EU research project HIPPOCRATES develops personalized therapy approaches for people with psoriatic arthritis”, www.fau.de/2021/07/news/wissenschaft/eu-projekt-soll-behandlungsmoeglichkeiten-der-psoriasis-arthritis-verbessern,(last accessed 25.09.2024).
- Pennington SR, et al: Multi-omics Approach to the Identification of Biomarkers for Progression from Psoriasis to Psoriatic Arthritis, Poster, MSACL 202414th Annual Conference & Exhibits, March 17-22, 2024. www.msacl.org, (last accessed 09/25/2024).
- Kampylafka E, et al: Disease interception with interleukin-17 inhibition in high-risk psoriasis patients with subclinical joint inflammation-data from the prospective IVEPSA study. Arthritis Res Ther 2019 Jul 26; 21(1): 178.
- Haberman RH, et al: Efficacy of guselkumab, a selective IL-23 inhibitor, in Preventing Arthritis in a Multicentre Psoriasis At-Risk cohort (PAMPA): protocol of a randomized, double-blind, placebo controlled multicentre trial. BMJ Open 2022 Dec 23; 12(12): e063650. doi: 10.1136/bmjopen-2022-063650.
- Merola JF, et al: A clinical review of structural damage in psoriatic arthritis for dermatologists: From pathogenesis to ongoing controversies. Journal of the American Academy of Dermatology 2024; 90(Issue 2) 349-357.
- Scher JU, et al: Preventing psoriatic arthritis: focusing on patients with psoriasis at increased risk of transition. Nat Rev Rheumatol 2019; 15(3): 153-166.
- Kimak A, et al: Psoriatic Arthritis: Development, Detection and Prevention: A Scoping Review. Journal of Clinical Medicine. 2023; 12(11): 3850.
www.mdpi.com/2077-0383/12/11/3850#,(last accessed 25.09.2024). - Swissmedic: Medicinal product information, www.swissmedicinfo.ch,(last accessed 26.09.2024).
DERMATOLOGIE PRAXIS 2024; 34(5): 24-26 (published on 28.10.24, ahead of print)