In a study involving dermatologists from the University Hospital Zurich, preoperative immunotherapy was shown to increase the likelihood of survival and a longer recurrence-free interval in melanoma patients. For patients, this brings justified hope for better therapeutic success and disease progression. However, the study is not yet complete with the three years of observation; a further evaluation is to follow after five years.
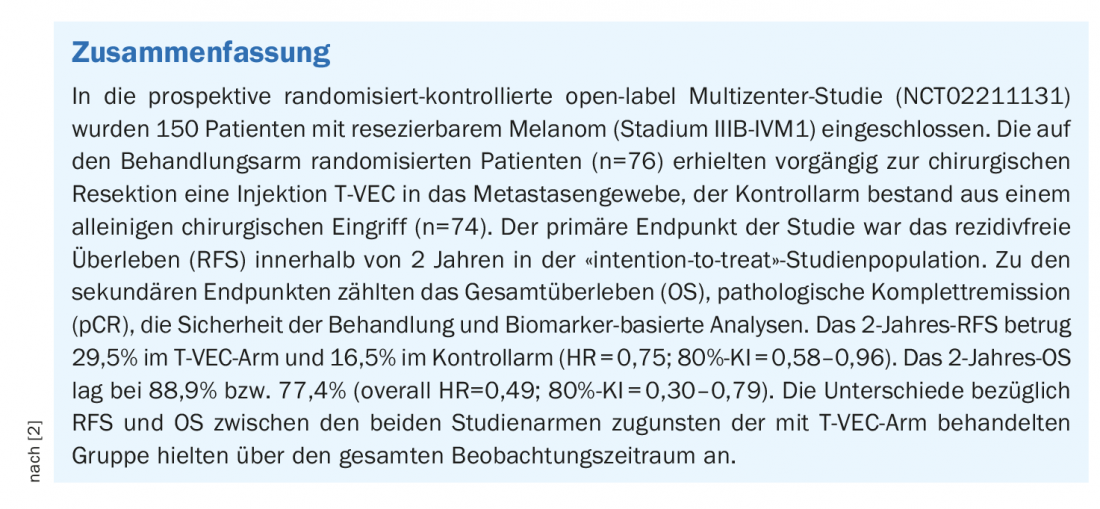
A prospective phase II clinical trial led by Prof. Reinhard Dummer, MD, University Hospital Zurich, investigated the use of talimogene laherparepvec (T-VEC) (box ) in patients with resectable melanoma [1,2]. The study included participants from 48 treatment centers in 9 countries. Patients were randomized to a study arm with T-VEC prior to surgical resection (n=76) vs. a control arm with resection alone (n=74). T-VEC was injected into the metastatic tissue.
High probability of a better course of the disease
“The study will have a lasting impact on the therapeutic approach to melanoma of the skin,” Prof. Dummer said. The analysis of the patient data shows for the three years of the observation period better values for the patients treated with immunotherapy before surgery than for those with resection alone, both for overall survival and for time to disease recurrence and survival without relapse. For example, the probability of survival among patients with preoperative immunotherapy within the three years was 83.2% vs. 71.6%; for a longer time without relapse, 46.5% vs. 31% [1,2]. The 2-year overall survival (OS, overal survival) rate was 88.9% in the T-VEC arm and 77.4% in the control arm, corresponding to a 51% reduction in mortality risk in favor of the T-VEC-treated group (HR=0.49) [2,3].

The probability of relapse-free survival (RFS) within 2 years was 29.5% in the T-VEC group and 16.5% in the control group, indicating a 25% reduced risk of relapse (HR=0.75). 33.7% of those who received preoperative immunotherapy remained free of distant metastases within 2 years, compared with 19.5% in the control group. The estimated 3-year RFS rate was 28.1% in the T-VEC arm and 16.9% in the control arm (HR 0.74; 80% CI: 0.57-0.95) [2,3].
|
“The results impressively demonstrate the potential of neoadjuvant therapy approaches and this preoperative immunotherapy in melanoma,” Prof. Reinhard Dummer, MD, Dermatology Clinic, University Hospital Zurich, summarized the results [1]. |
The mean age of study participants at baseline was 63.5 years (range 34-85 J.) in the T-VEC group and at 59.0 years (range 21-85 J.) in the control arm. 64.5% and 63.5% of subjects were male, respectively. In the T-VEC arm, 13 patients (17.1%) achieved pathologic complete remission (pCR). Six patients in this subgroup had stable disease and two had progressive disease prior to surgical intervention [2,3].
Literature:
- “Skin cancer: immunotherapy before surgery increases the likelihood of better disease outcome,” University Hospital Zurich, Oct. 07, 2021.
- Dummer R, et al: Neoadjuvant talimogene laherparepvec plus surgery versus surgery alone for resectable stage IIIB-IVM1a melanoma: a randomized, open-label, phase 2 trial. Nature Medicine 2021; 27: 1789-1796.
- Cancer Therapy Advisor, www.cancertherapyadvisor.com/home/cancer-topics/skin-cancer/melanoma-neoadjuvant-tvec-improves-survival-treatment-risk (last accessed Apr. 11, 2022).
- European Medicines Agency, www.ema.europa.eu/en/documents/product-information/imlygic-e par-product-information_en.pdf, last accessed, 04/11/2022).
DERMATOLOGIE PRAXIS 2022; 32(2): 48