Stroke is a leading cause of disability. Cognitive impairment occurring after stroke has been rather neglected for many years. The review focus on the incidence and prevalence of cognitive decline following stroke, on the main predisposing factors, on imaging factors and on potential candidate biomarkers.
Stroke is a leading cause of disability [1]. While most of research and interventions focused on physical disabilities [2], cognitive impairment occurring after stroke has been rather neglected for many years [3,4]. It is presently known that even minor strokes may affect daily functioning and cognition and will consequently influence quality of life [5]. Ischemic stroke can facilitate the onset of vascular dementia as well as aggravate pre-existing cognitive decline. The onset of cognitive decline may become manifest immediately following the onset of ischemic stroke, but often there is a delay in the development of cognitive decline after a stroke [6]. Both neurodegenerative and vascular mechanisms are activated and probably result in overlapping processes within the neurovascular unit [7]. In the present review, we will focus on the incidence and prevalence of cognitive decline following stroke, on the main predisposing factors, on imaging factors and on potential candidate biomarkers.
Epidemiology
Stroke survivors are at increased risk of developing cognitive impairment. Reported estimates of the prevalence of dementia are consistent across various studies: 10% of the patients present dementia before first stroke, 10% develop new dementia after first stroke, and more than a third have dementia after recurrent stroke [8,9].
The strong association of post-stroke dementia with multiple strokes highlight the central causal role of stroke and, thus, the likely effect of optimum acute stroke care and secondary prevention in reducing the burden of dementia [8]. The prevalence of cognitive impairment in stroke survivors varies depending on the setting, the population, the exclusion criteria (pre-stroke dementia, recurrent stroke, aphasia), the criteria used for the diagnosis of cognitive impairment and the time interval since stroke [10]. The prevalence of post-stroke dementia in the first year after stroke ranges from 7% in population-based studies of first-ever stroke excluding pre-stroke dementia to 41% in hospital-based studies including recurrent stroke and pre-stroke dementia [8). The risk of post-stroke dementia was found to be highest in the first months after stroke, which might partially be due to unrecognized cognitive impairment before stroke [10]. After the initial post-stroke incidence of dementia, the cumulative incidence increases linearly at a rate of 3% and 1,7% per year in hospital-based and population-based studies, respectively [11]. Few studies used long-term outcomes: the longest observational period was 25 years in a population based study finding a cumulative incidence of post-stroke dementia of 48% at year 25 [12].
Cognitive impairment may also occur after TIA. In a recent systematic review including 1167 patients, the prevalence of post-TIA mild cognitive impairment ranged from 29 to 68%. Severe cognitive impairment was found in 8–22% of patients. Studies using a cognitive screening instrument and those performed shortly after TIA or several years later, reported the highest frequencies of impairment [13].
Definitions
Vascular dementia is the second most common cause of cognitive decline after Alzheimer’s disease and includes post-stroke dementia. Therefore, the terms PSD and vascular dementia are not synonymous. Vascular dementia represents a concept which includes not only multiple cortical and/or subcortical infarcts, but also strategic single infarcts, non-infarction white matter lesions, hemorrhages, and hypo perfusion as possible causes of dementia. Furthermore, it must be emphasized that not all cases of post-stroke dementia are of vascular origin. Accordingly, the term of post-stroke dementia (PSD) is used for any dementia which develops following a clinical cerebrovascular event. In this way, the term PSD does not suggest a particular neuropathological process. Furthermore, recognizing the pre-stroke cognitive state is essential to allow appropriate classification. A patient with pre-existing cognitive impairment who presents a minor stroke should not be labeled as PSD. Furthermore, the time assessment of cognitive impairment is another relevant diagnostic factor. Acute deficiencies in cognitive test scores are often observed following a stroke and retesting after several weeks often reveals improvements [10]. Therefore, it is recommended that the final diagnosis of PSD should be delayed to at least six months after the event.
It should be further noticed that the term «post-stroke» includes not only strokes and minor strokes but also transient ischemic attacks (TIA), as emerging evidence suggests that TIAs may also be associated with adverse cognitive prognosis [13].
Screening tests
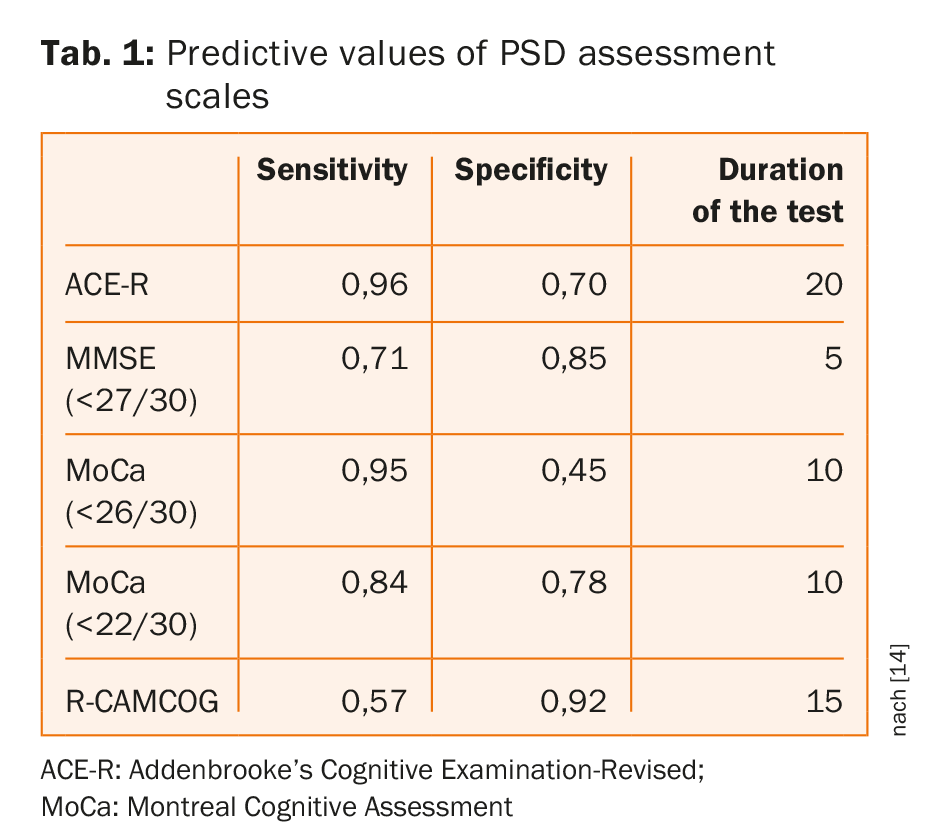
Commonly used cognitive screening tools have similar accuracy for detection of dementia/multi-domain impairment with no clearly superior test and no evidence that screening tools with longer administration times perform better [14]. As shown on table 1, MoCA at usual threshold offers short assessment time with high sensitivity but at cost of specificity; adapted cutoffs improved specificity without sacrificing sensitivity [14].
Risk factors for post-stroke dementia
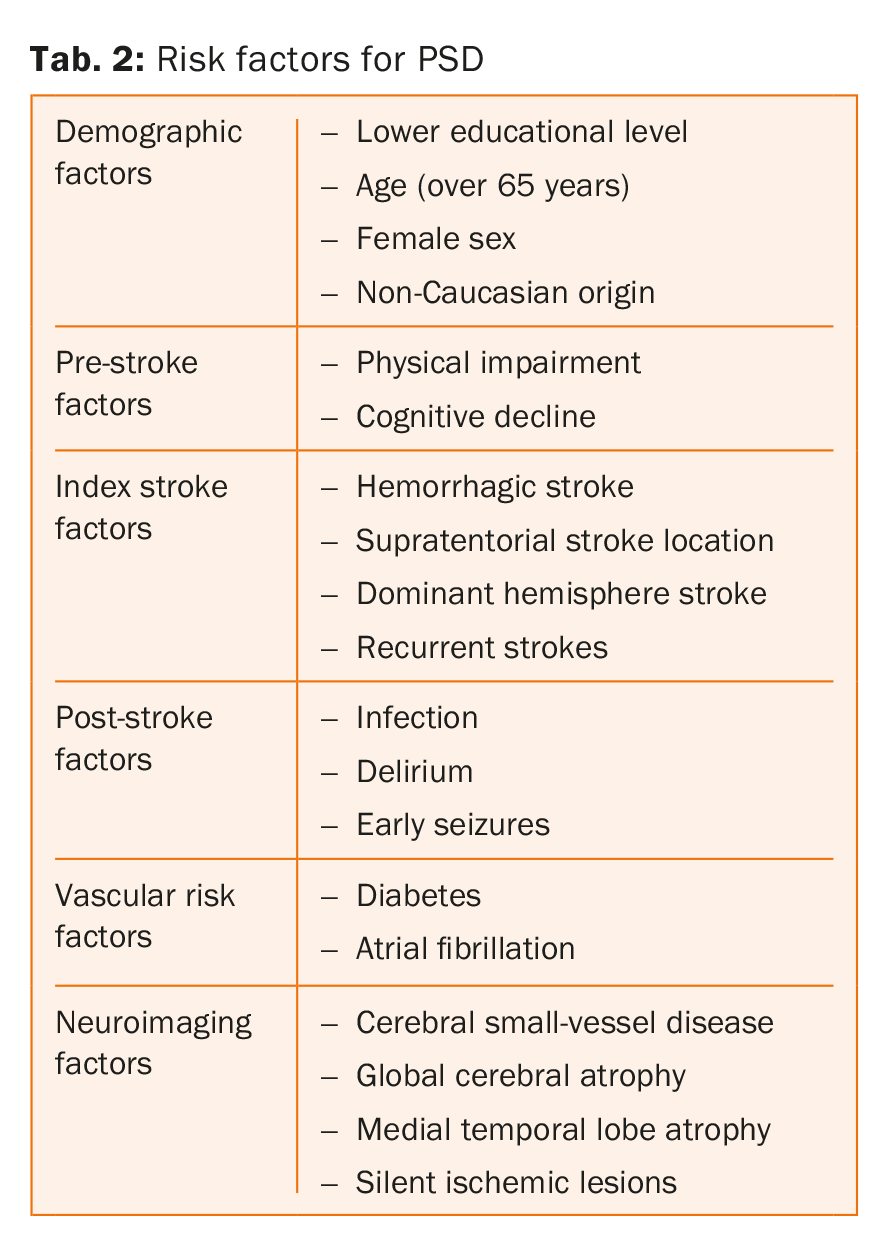
The main risk factors for post-stroke dementia are listed on table 2. In fact these parameters act on distinct levels, some rather on the pre-stroke whereas others on the post-stroke condition. In a systematic review and meta-analysis including 7511 patients, Pendlebury and colleagues showed that medial temporal lobe atrophy, female sex, and a family history of dementia were strongly associated with pre-stroke dementia, whereas the characteristics and complications of the stroke and the presence of multiple lesions in time and place were more strongly associated with post-stroke dementia [8].
Noteworthy is also the fact that vascular risk factors such as diabetes and atrial fibrillation are associated with an increased risk of post-stroke dementia independently from pre-stroke cognitive impairment, whereas this was not found for two other well established risk factors, hypertension and smoking [8,15-18]. This finding however is in contradiction with the results of other studies showing that arterial hypertension is an important determinant of cognitive impairment [19,20]. Finally, evidence of whether lifestyle related vascular risk factors such as physical inactivity and unhealthy diet are also independent risk factors for PSD is missing [21].
Stroke subtypes
It is generally thought that lacunar strokes might be less likely to affect cognition than more severe, larger cortical strokes, however lacunar strokes are usually associated with cerebral small vessel disease, a common cause of cognitive impairment and dementia, especially in the elderly [22]. In a recent meta-analysis, Makin and coworkers, compared the incidence of cognitive impairment accordingly to different stroke subtypes [23]. The authors demonstrated among 7575 patients, including 2860 with lacunar stroke, that 24% had mild cognitive impairment (MCI) or PSD. The prevalence of dementia after lacunar stroke (six studies, n=1421) was 20% (95% CI 9 to 33) and the incidence of MCI or dementia (four studies, n=275) was 37% (95% CI 23 to 53). The authors concluded that cognitive impairment appeared to be common after lacunar strokes despite their small size, suggesting that associated SVD may increase their impact. No significant difference regarding the prevalence of PSD between lacunar and non-lacunar strokes was observed.
Neuroimaging
Neuroimaging is an important diagnostic tool in PSD. Magnetic resonance imaging (MRI) is the key neuroimaging modality yielding a high sensitivity and specificity for detecting pathological changes, including small vessel disease. For the diagnosis of small vessel disease and post-stroke cognitive decline MRI should be used with several sequences. Standards for neuroimaging are recommended with a widely accepted terminology permitting comparison of findings [26].
Positron emission tomography (PET) allows imaging of the localized and/or diffuse metabolic disturbances responsible for cognitive impairment and dementia, and is effective in differentiating vascular from degenerative dementia, such as Alzheimer’s disease (decreased metabolism in temporo-mesial, temporo-parietal cortex and posterior cingulum, preserved metabolism in frontal and visual cortex, in central region and basal ganglia). It can also detect inflammatory changes and their interaction with amyloid depositions for the development of mixed dementias after stroke [27].
Imaging predictors
Some MRI imaging features have a predictive value regarding occurrence of PSD. In a recent study, 294 patients with SVD were evaluated three to five years after initial presentation of a subcortical stroke of lacunar type [28]. At follow-up, vascular cognitive impairment (VCI) of any type was detected in 188 (63,9%) of SVD patients, with 65 (22,1%) meeting criteria for vascular dementia. Multivariate logistic regression analysis adjusted by age and gender identified overall severity of white matter hyper-intensities (tARWMC HR 1,42, 95% CI 1,01-2,00; p0,043) and total number of lacunar infarcts (HR 3,06, 95% CI 1,71-5,50, p < 0,001) as independent predictors of cognitive decline. Another study compared the prognostic value of medial temporal lobe atrophy and features of SVD in a cohort of 234 patients with stroke or TIA [29]. The relationship between radiological features suggestive of Alzheimer’s disease and SVD was explored and the association of each of these features with cognitive status at one year was investigated. SVD features were independently associated with MTA (p<0,001). After adjusting for age, sex, disability after stroke, hypertension and diabetes mellitus, medial temporal atrophy was the only radiological feature independently associated with cognitive impairment, defined using thresholds of mini mental state (MMSE) ≤26 (odds ratio 1,94; 95% CI1,28–2,94) and MMSE ≤23 (odds ratio 2,31; 95% CI1,48–3,62).
Biomarkers
Biomarkers for PSD may include metabolic, genetic and inflammatory mediators. The e4 allele of apolipoprotein E (APOE4) is a well-known risk factor for Alzheimer’s disease [30,31). APOE4 is also associated with cardiovascular disease and brain infarcts [31]. Conflicting data exist regarding the association of APOE4 polymorphism with vascular dementia and PSD [32–35]. The renin-angiotensin system, via both metabolic and vascular effects, is reported to be involved in the pathogenesis of dementia [36,37]. Angiotensin-converting enzyme (ACE) is one of the enzymes in the renin-angiotensin system. Patients with ACE genotype have high levels of ACE in the plasma and are at greater risk for cardiovascular comorbidity [38–40]. Previous studies showed an association between ACE allele and cognitive decline. This effect may be stronger in the presence of APOE4 [41,42]. However, studies investigating ACE as a predictor for post-stroke cognitive decline ended with conflicting data [42,43]. Other biomarkers such as B secretase enzyme (BACE1) and receptor levels for advanced glycation end-product (sRAGE) have been suggested to correlate with cognitive impairment immediately after stroke (assessed two weeks after stroke) [39]. Homocysteine, vitamin B12 and folic acid levels were linked to cognitive decline and stroke, and might play a role in PSD. However, in the VITATOPS trial supplementation with B vitamins had no effect on the incidence of cognitive impairment or cognitive decline [44]. An excessive inflammatory environment in the brain could aggravate post ischemic damage. Therefore, individuals with elevated inflammatory response to the ischemic insult may be more vulnerable to further tissue damage and for developing PSD.
Systemic inflammation and inflammatory markers are known to be associated with cognitive impairment [45], particularly with degenerative dementia like Alzheimer’s disease [46–48]. Longitudinal studies showed a correlation between higher baseline concentration of interleukin 6 (IL-6) [48] and C-reactive protein (CRP) [49] and cognitive decline. However, the association between inflammation and PSD is not yet established. Several recent studies have investigated the relationship between inflammatory markers and PSD. Erythrocyte sedimentation rate (ESR) [50], CRP and IL-6 [51] were suggested as predictors of PSD.
Pharmacological treatment and prevention
There are various pharmacological interventions for the prevention of post-stroke cognitive decline. Long-term blood pressure lowering post-stroke with perindopril was associated with reduced cognitive decline and a trend to less dementia in the PROGRESS trial [52].
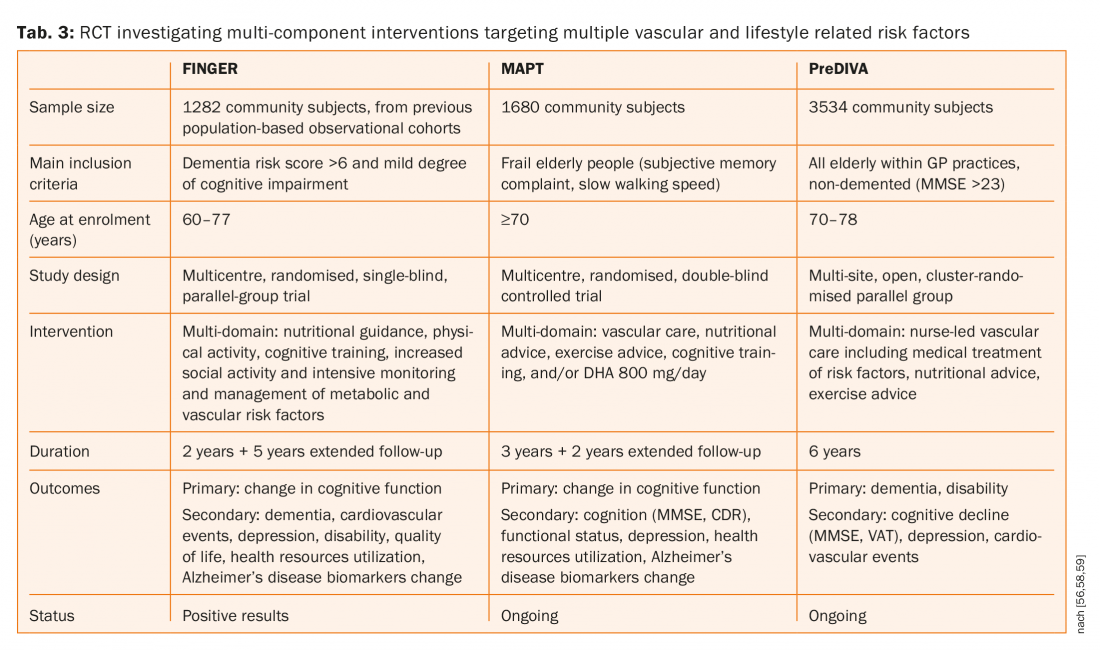
Furthermore, statins have been shown to reduce both first and recurrent stroke, but neither simvastatin nor pravastatin had any influence on cognition [53,54]. Three large randomised controlled trials (FINGER, MAPT and preDIVA, tab. 3) investigating multi-component interventions targeting multiple vascular and lifestyle related risk factors versus general health advice (control group) to prevent cognitive decline and dementia [55–58]. One of these three studies (FINGER) published their results and suggested that a multi-domain intervention could improve or maintain cognitive functioning in at-risk elderly people [55,56]. There were 1260 patients randomly assigned to the intervention group (n=631) or control group (n=629). 591 (94%) participants in the intervention group and 599 (95%) in the control group had at least one post-baseline assessment and were included in the modified intention-to-treat analysis. Estimated mean change in neuropsychological test battery score at two years was 0,20 in the intervention group and 0·16 in the control group. Between-group difference in the change of neuropsychological test battery total score per year was 0,022 (95% CI 0,002–0,042; p=0,030).
Conclusion
An important proportion of patients will suffer from dementia or milder forms of cognitive deterioration after a stroke or even a TIA. A certain number of clinical and radiological parameters may predict the occurrence of PSD. Although the risk is reported to be highest in the immediate post-stroke period, it still will remain high even after several years. The presence of this delay between time of stroke and onset of dementia enables further the use of a therapeutic time window for intervention. Pharmacological studies showed that long-term blood pressure lowering post-stroke was associated with reduced cognitive decline. Recently a large randomised controlled trial showed that a multi-domain intervention could improve or maintain cognitive functioning in at-risk elderly people. These results further suggest the positive influence of multi-component interventions targeting multiple vascular and lifestyle related risk factors on the occurrence of post-stroke dementia.
Take-Home-Messages
- Stroke survivors are at increased risk of developing cognitive impairment.
- Post-stroke dementia (PSD) is associated with several factors indicating on one hand a reduced cognitive reserve including pre-stroke cognitive decline, premorbid disability, white matter disease and cerebral atrophy and on the other specific stroke aspects.
- Magnetic resonance imaging (MRI) is the key neuroimaging modality.
- Although a large number of biomarkers have been proposed for PSD, no specific parameter has been proven yet to robustly predict PSD.
- Pharmacological studies showed that long-term blood pressure lowering post-stroke was associated with reduced cognitive decline.
- A large randomised controlled trial recently suggested that a multi domain intervention could improve or maintain cognitive functioning in at-risk elderly people.
References:
- Strong K, Mathers C, et al.: Preventing stroke: saving lives around the world. Lancet Neurol. 2007; 6: 182–187.
- Lees R, Fearon P, Harrison JK, et al.: Cognitive and mood assessment in stroke research: focused review of contemporary studies. Stroke. 2012; 43: 1678–1680.
- McKevitt C, Fudge N, et al.: Self-reported long term needs after stroke. Stroke. 2011; 42: 1398–1393.
- Pollock A, St George B, et al.: Top ten research priorities relating to life after stroke. Lancet Neurol. 2012; 11: 209.
- Fride Y, Adamit T, et al.: What are the correlates of cognition and participation to return to work after first ever mild stroke? Top Stroke Rehabil. 2015;22(5): 317–325.
- Ballard C, Rowan E, et al.: Prospective follow-up study between 3 and 15 months after stroke: improvements and decline in cognitive function among dementia-free stroke survivors >75 years of age. Stroke 2003; 34: 2440–2444.
- Kalaria RN: Cerebrovascular disease and mechanisms of cognitive impairment: evidence from clinicopathological studies in humans.Stroke 2012; 43: 2526–2534.
- Pendlebury ST, Rothwell PM: Prevalence, incidence, and factors associated with pre-stroke and post-stroke dementia: a systematic review and meta-analysis. Lancet Neurol. 2009; 8: 1006–1018.
- Pendlebury ST, Chen PJ, et al.: Oxford Vascular Study. Methodological factors in determining risk of dementia after transient ischemic attack and stroke: (I) impact of baseline selection bias. Stroke. 2015; 46: 641–646.
- Henon H, Pasquier F, Leys D: Poststroke dementia. Cerebrovasc Dis 2006; 22: 61–70.
- Mijajlović MD, Pavlović A, et al.: Post-stroke dementia – a comprehensive review. BMC Med. 2017 Jan 18; 15(1):11.
- Kokmen E, Whisnant JP, et al.: Dementia after ischemic stroke: a population- based study in Rochester, Minnesota (1960–1984). Neurology 1996; 46: 154–159.
- Van Rooij FG, Kessels RP, et al.: Cognitive impairment in transient ischemic attack patients: a systematic review. Cerebrovasc Dis. 2016; 42(1–2): 1–9.
- Lees R, Selvarajah J et al.:Test accuracy of cognitive screening tests for diagnosis of dementia and multidomain cognitive impairment in stroke. Stroke. 2014 Oct; 45(10): 3008-3018.
- Gottesman RF, Hillis AE: Predictors and assessment of cognitive dysfunction resulting from ischaemic stroke. Lancet Neurol 2010; 9: 895–905.
- Pasquier F, Hénon H, Leys D: Risk factors and mechanisms of post-stroke dementia. Rev Neurol. 1999; 155(9): 749–753.
- Chaudhari TS, Verma R, et al.: Clinico-radiological predictors of vascular cognitive impairment (VCI) in patients with stroke: a prospective observational study. J Neurol Sci. 2014 May 15; 340(1-2): 150-158.
- Arba F, Quinn T, et al.: VISTA Collaboration. Determinants of post-stroke cognitive impairment: analysis from VISTA. Acta Neurol Scand. 2017 Jun; 135(6): 603–607.
- Iulita MF, Girouard H: Treating Hypertension to Prevent Cognitive Decline and Dementia: Re-Opening the Debate. Adv Exp Med Biol. 2017; 956: 447–473.
- Kalaria RN: Risk factors and neurodegenerative mechanisms in stroke related dementia. Panminerva Med. 2012 Sep; 54(3):139–148.
- Teuschl Y, Matz K, Brainin M: Prevention of post-stroke cognitive decline: a review focusing on lifestyle interventions. Eur J Neurol. 2013 Jan; 20(1): 35–49.
- Wardlaw JM, Smith EE, et al.: Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol 2013; 12: 822–838.
- Makin SD, Turpin S, et al.: Cognitive impairment after lacunar stroke: systematic review and meta-analysis of incidence, prevalence and comparison with other stroke subtypes. J Neurol Neurosurg Psychiatry. 2013 Aug; 84(8): 893-900.
- Schmidt R, Ropele S, et al.: Diffusion-weighted imaging and cognition in the leukoariosis and disability in the elderly study. Stroke 2010; 41: e402–e408.
- Blair GW, Hernandez MV, et al.: Advanced Neuroimaging of Cerebral Small Vessel Disease. Curr Treat Options Cardiovasc Med. 2017 Jul; 19(7): 56.
- Wardlaw JM, Smith EE, et al.: Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013; 12: 822–838.
- Heiss W-D, Zimmermann-Meinzingen S: PET imaging in the differential diagnosis of vascular dementia. J Neurol Sci 2012; 322: 268–273.
- Pavlovic AM, Pekmezovic T, et al.: Baseline predictors of cognitive decline in patients with cerebral small vessel disease. J Alzheimers Dis. 2014; 42 Suppl 3: 37–43.
- Arba F, Quinn T, et al.: VISTA. Collaboration.Cerebral small vessel disease, medial temporal lobe atrophy and cognitive status in patients with ischaemic stroke and transient ischaemic attack. Eur J Neurol. 2017 Feb; 24(2): 276-282.
- Bu G: Apolipoprotein E and its receptors in Alzheimer’s disease: pathways, pathogenesis and therapy. Nat Rev Neurosci 2009; 10: 333–344.
- Davidson Y, Gibbons L et al.: Apolipoprotein E epsilon4 allele frequency in vascular dementia. Dement Geriatr Cogn Disord 2006; 22: 15–19.
- Hsiung G-YR, Sadovnick AD, Feldman H: Apolipoprotein E epsilon4 genotype as a risk factor for cognitive decline and dementia: data from the Canadian Study of Health and Aging. CMAJ 2004; 171: 863–867.
- Mortimer JA, Snowdon DA, Markesbery WR: The effect of APOE-epsilon4 on dementia is mediated by Alzheimer neuropathology. Alzheimer Dis Assoc Disord 2009; 23: 152–157.
- Rippon GA, Tang MX, et al.: Familial Alzheimer disease in Latinos: interaction between APOE, stroke, and estrogen replacement. Neurology 2006; 66: 35–40.
- Jin YP, Østbye T, et al.: Joint effect of stroke and APOE 4 on dementia risk: the Canadian Study of Health and Aging. Neurology 2008; 70: 9–16.
- Qian L, Ding L, et al.: Early biomarkers for post-stroke cognitive impairment. J Neurol 2012; 259: 2111–2118.
- Kolsch H, Jessen F, et al.: ACE I/D polymorphism is a risk factor of Alzheimer’s disease but not of vascular dementia. Neurosci Lett 2005; 377: 37–39.
- Hassan A, Lansbury A, et al.: Angiotensin converting enzyme insertion/deletion genotype is associated with leukoaraiosis in lacunar syndromes. J Neurol Neurosurg Psychiatry 2002; 72: 343–346.
- Szolnoki Z, Maasz A, et al.: Coexistence of angiotensin II type-1 receptor A1166C and angiotensin converting enzyme D/D polymorphism suggests susceptibility for small-vessel-associated ischemic stroke. Neuromolecular Med 2006; 8: 353–360.
- Bartres-Faz D, Junque C, et al.: Angiotensin II converting enzyme polymorphism in humans with age-associated memory impairment: relationship with cognitive performance. Neurosci Lett 2000; 290: 177–180.
- Richard F, Berr C, et al.: Effect of the angiotensin I-converting enzyme I/D polymorphism on cognitive decline. The EVA Study Group. Neurobiol Aging 2000; 21: 75–80.
- Bour AMJ, Rasquin SMC, et al.: The effect of the APOE-epsilon4 allele and ACE-I/D polymorphism on cognition during a two-year follow-up in first-ever stroke patients. Dement Geriatr Cogn Disord 2010; 29: 534–542.
- Bour AMJJ, Rasquin SMC, et al.: The effect of the APOE-epsilon4 allele and ACE-I/D polymorphism on cognition during a two-year follow-up in first-ever stroke patients. Dement Geriatr Cogn Disord 2010; 29: 534–542.
- Baum L, Chen X, et al.: Polymorphisms and vascular cognitive impairment after ischemic stroke. J Geriatr Psychiatry Neurol 2007; 20: 93–99.
- Hankey GJ, Ford AH et al.: Effect of B vitamins and lowering homocysteine on cognitive impairment in patients with previous stroke or transient ischemic attack: a prespecified secondary analysis of a randomised, placebo-controlled trial and meta-analysis. Stroke 2013; 44: 2232–2239.
- Karlsson H, Ahlborg B, et al.: Association between erythrocyte sedimentation rate and IQ in Swedish males aged 18–20. Brain Behav Immun 2010; 24: 868–873.
- Craft S: The role of metabolic disorders in Alzheimer disease and vascular dementia: two roads converged. Arch Neurol 2009; 66: 300–305.
- Eikelenboom P, van Exel E, et al.: Neuroinflammation – an early event in both the history and pathogenesis of Alzheimer’s disease. Neurodegener Dis 2010; 7: 38–41.
- Schram MT, Euser SM et al.: Systemic markers of inflammation and cognitive decline in old age. J Am Geriatr Soc 2007; 55: 708–716.
- Hoth KF, Haley AP, et al.: Elevated C-reactive protein is related to cognitive decline in older adults with cardiovascular disease. J Am Geriatr Soc 2008; 56: 1898–1903.
- Kliper E, Bashat DB et al.: Cognitive decline after stroke: relation to inflammatory biomarkers and hippocampal volume. Stroke 2013; 44: 1433–1435.
- Rothenburg LS, Herrmann N, et al.: The relationship between inflammatory markers and post stroke cognitive impairment. J Geriatr Psychiatry Neurol 2010; 23: 199–205.
- Tzourio C, Anderson C, et al.: Effects of blood pressure lowering with perindopril and indapamide therapy on dementia and cognitive decline in patients with cerebrovascular disease. Arch Intern Med 2003; 163: 1069–1075.
- Collins R, Armitage J: Heart Protection Study Collaborative Group. Effects of cholesterol-lowering with simvastatin on stroke and other major vascular events in 20536 people with cerebrovascular disease or other high-risk conditions. Lancet 2004; 363: 757–767.
- Shepherd J, Blauw GJ, et al.: Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet 2002; 360: 1623–1630.
- Kivipelto M, Solomon A, et al.: The Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER): study design and progress. Alzheimers Dement 2013; 9: 657–665.
- Ngandu T, Lehtisalo J, et al.: A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet. 2015 Jun 6; 385(9984): 2255-2263.
- Carrie I, van Kan GA, et al.: Recruitment strategies for preventive trials. The MAPT study (MultiDomain Alzheimer Preventive Trial). J Nutr Health Aging 2012; 16: 355–359.
- Richard E, Van den Heuvel E, et al.: Prevention of dementia by intensive vascular care (PreDIVA): a cluster-randomised trial in progress. Alzheimer Dis Assoc Disord 2009; 23: 198–204.
InFo NEUROLOGIE & PSYCHIATRIE 2018; 16(1): 31–37