Any surgery can cause problems with the circulation, heart or lungs postoperatively. The prevention of such complications and their treatment is of great importance, because otherwise the perioperative risk increases significantly. This requires a thoracic surgery treatment team to be aware of intraoperative and postoperative risks and to be proficient in the management of complications that may arise.
Any surgery can cause problems with the circulation, heart or lungs postoperatively. The prevention of such complications and their treatment is of great importance, because otherwise the perioperative risk increases significantly. This requires a thoracic surgery treatment team to be aware of intraoperative and postoperative risks and to be proficient in the management of complications that may arise.

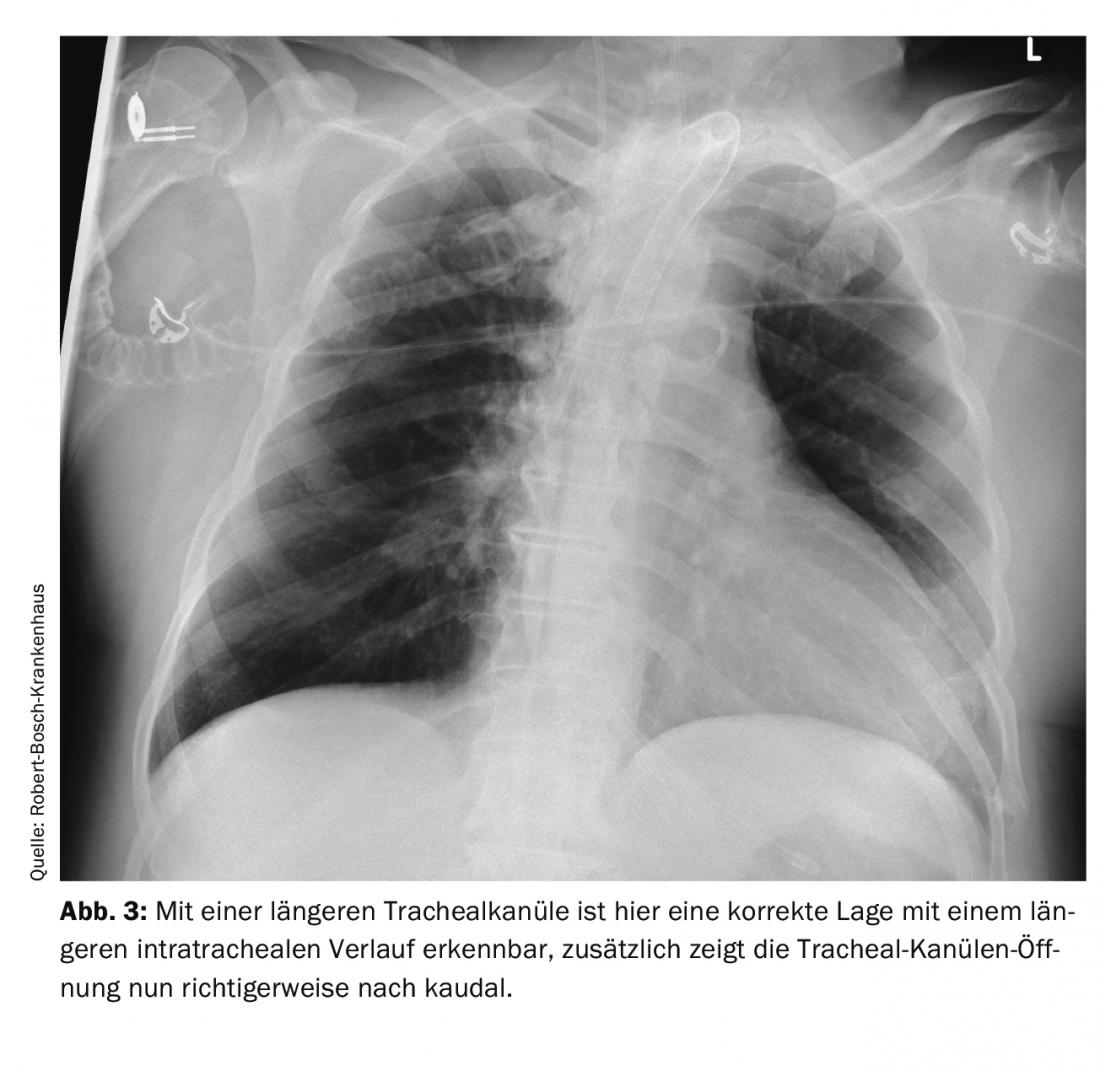
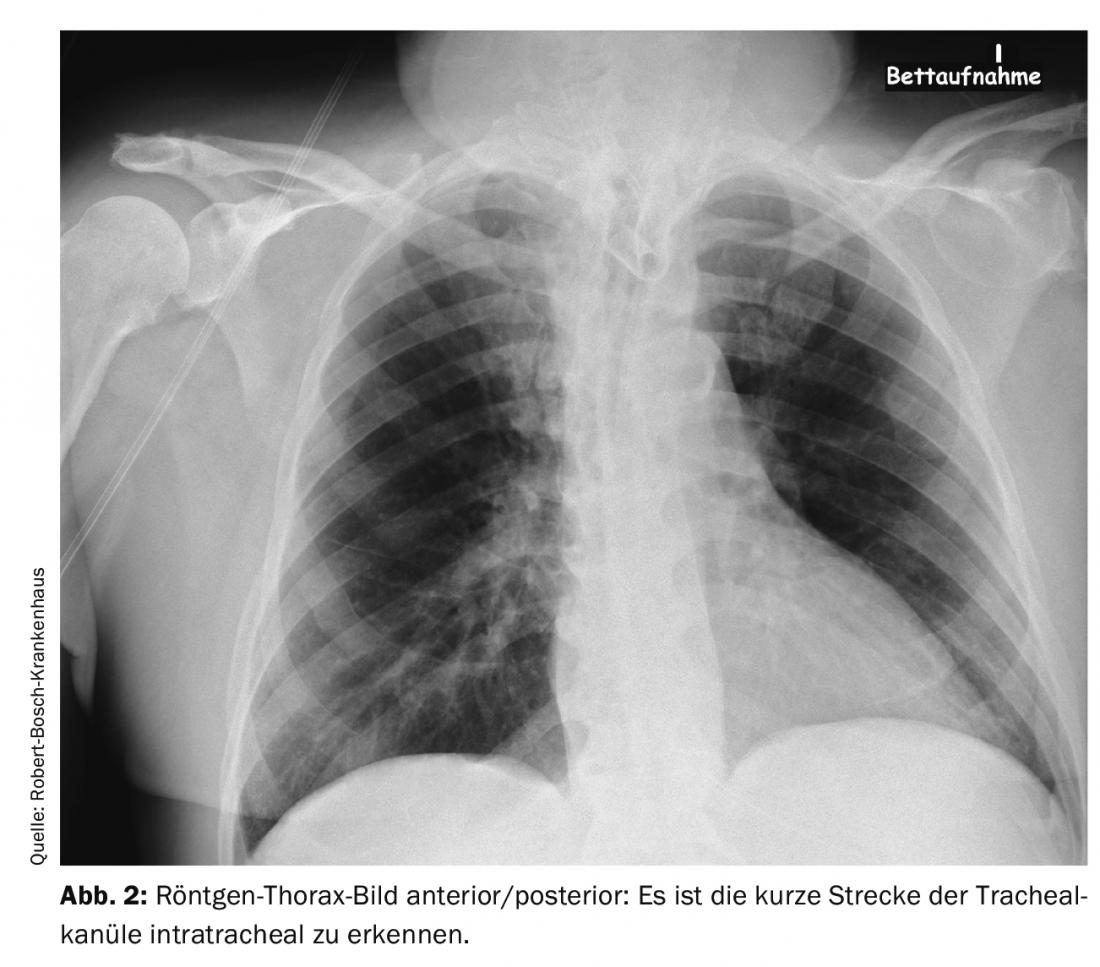
Even a relatively minor procedure, such as the creation of a tracheostoma, can leave a patient in vital danger after the procedure: In a 66-year-old patient, a dilated tracheostomy was first attempted. Since this was unsuccessful, a plastic tracheostomy was performed. Shortly thereafter, the patient was transferred to another hospital. Over the next few days, he experienced recurrent ventilatory difficulties and hypercapnia. The cause was thought to be bronchospasm and stress-related decompensation due to reduction of sedation for weaning from the ventilator. Diagnostic imaging was performed several times. The cause was an improperly positioned tracheal cannula. The cannula was too short and therefore lay only with the tip intratracheal and with the opening directly at the posterior tracheal wall. This resulted in intermittent airway obstruction. In the X-ray – thorax image and CT scan of the thorax, if you know the signs, you can see this malposition. It can be seen in the images in Figure 1 and 2 the position of the tracheal cannula opening directly on the tracheal wall with the only short distance intratracheal and after correction by a longer tracheal cannula in Figure 3. The easiest way to detect this problem is by tracheoscopic inspection with a bronchoscope. In such a situation, even an easily correctable problem can lead to a serious disorder with hypoxia and hypercapnia if care is not taken with the details in the diagnosis and thus the real problem of the respiratory disorder is not recognized.
To reduce the risk of postoperative complications, the following should always be checked when a patient is transferred to a monitoring or intensive care unit after surgery: consciousness, depth of sedation if necessary, whether protective reflexes are present in extubated patients, spontaneous breathing or ventilation parameters, position of the tube or tracheal cannula, lateral ventilation of the lungs, position and flow rate of chest drains, suction setting on the drains, and circulatory parameters. A procedure according to a standard is useful here, e.g. a sequence based on the ABCDE scheme for checking vital signs. A handover with operative measures documented in writing, the intraoperative peculiarities and the problems encountered is also recommended. Here, a handover protocol with the SBAR concept is recommended for error prevention [1]. The suction setting on the chest drains is of particular importance. When air is lost from the lung parenchyma, negative pressure at the drains of the thoracic cavity is necessary to prevent pneumothorax, but suction set too high may increase air loss. During a pneumectomy, the setting of negative suction (greater than minus five mbar) is contraindicated, as this negative pressure can otherwise lead to displacement of the mediastinum (similar effect to tension pneumothorax) and thus to serious cardiopulmonary complications. Postoperative hemorrhage can be detected by the amount of drainage loss, by hemoglobin determination in the patient’s blood and, if necessary, in the drainage fluid, by sonographic imaging of the pleural space, and, in advanced stages, by a circulatory reaction due to the lack of volume. If these measures do not lead to sufficient information, a CT scan of the thorax often helps. In addition to monitoring the coagulation parameters and, if necessary, taking measures to improve coagulation, it is useful to inform the surgical department/thoracic surgery about a possibly necessary surgical revision. Other, rarer surgically induced complications such as bronchial stump insufficiency (recognizable by higher air fistula volumes via the chest drains) or flap torsion (first sign often an atelectasis in the X-ray thorax image) can often be verified with bronchoscopy and, if necessary, additionally a CT scan (with contrast medium for vascular imaging) and require surgical revision.
Pneumectomy with highest risk perioperatively
Respiratory and cardiac dysfunction is more common perioperatively during major thoracic surgery. For example, after pneumonectomy, the risk of myocardial infarction or cardiovascular death within the first 30 days after surgery is greater than 5% [2]. It is therefore advisable to clarify the patient’s cardiopulmonary risk factors before surgery and always perform a risk assessment. For videothoracoscopically assisted lobectomy, ASA classification has been described as a helpful method in this regard [3]. For general assessment of risk preoperatively, history, physical examination, laboratory values (with blood gas analysis), and imaging (chest x-ray or CT scan) are helpful. A resting ECG rarely provides new information preoperatively, but it can be very helpful as a comparison in postoperative problems caused by arrhythmias or ischemia. Functional examinations such as an exercise electrocardiogram, spiroergometry, a 6-minute walk test, estimation of the metabolic equivalent, and an indication of how many floors are possible while walking have a much better significance than measured so-called resting values. The planned surgery, videothoracoscopic procedure or thoracotomy, and how many segments of the lung are resected are additional factors in assessing perioperative risk. Postoperative lung function after resections can be estimated using the following formula: preoperative functional value (e.g., FEV1) × number of lung segments postoperatively / number of lung segments preoperatively [4].
Atrial fibrillation occurs with a frequency of 13 – 46% during major thoracic surgery [5]. In terms of surgery, the highest incidence is with pneumectomy. It is also a factor that increases the overall risk of surgery. Therapy depends on the effect on the patient’s circulation. The more unstable the circulation, the more likely it is that cardioversion is reasonable and necessary for new-onset atrial fibrillation. Medication-wise, amiodarone is most effective for conversion of cardiac rhythm. If pure rate control is the goal, beta blockers and digitalis are treatment options. In addition, it is always advisable to check the electrolytes (especially the potassium level) and, if necessary, to substitute potassium and magnesium. There are several approaches to prophylactic drug therapy, and beta-blockers and amiodarone have been shown to be the most effective.
Central venous saturation above 70% if possible
Intraoperative or postoperative heart failure should be diagnosed quickly. Intraoperatively, transesophageal echocardiography (TEE) may be helpful; postoperatively, semi-invasive systems such as monitoring with the Picco® system or trans-thoracic echocardiography (TTE) and, in some circumstances, a TEE scan may also be useful. Echocardiography also provides good detection of hemodynamically effective pericardial effusion and pericardial tamponade. Treatment of severe heart failure (Cardiac Index less than 1.5 l/min/m2 KÖF) is with catecholamines, phosphodiestherase-3 inhibitors, or levosimedan. Right heart failure is usually more difficult to treat than left heart failure. In right heart failure, increased pulmonary vascular resistance may be the problem; therapy with, for example, sildenafil or inhaled epoprostenol may be helpful. If the patient’s volume status is not clearly evident, a TTE examination or sonographic imaging of the inferior vena cava may be necessary. Sonographic measurements, TTE and TEE examinations are methods dependent on the experience of the investigator and should be available for extended lung resections. In addition, semi-invasive circulatory monitoring techniques can be used to make statements about volume status; for example, in the presence of sinus rhythm, stroke volume variance can be used to assess volume [6]. A simple laboratory value for estimating an adequate oxygen supply is the measurement of central venous saturation; this value should be above 70% (with a normal hemoglobin value) if possible. Postoperatively, monitor volume status by balancing imports and exports. Too much volume primarily impairs pulmonary function; too little intravascular volume leads to circulatory problems and, secondarily, organ dysfunction. Usually the kidneys are affected first, and in pronounced volume deficiency situations there is an increase in lactate levels (usually due to increased anaerobic metabolism or, less commonly, decreased breakdown in the liver).
Intraoperative bleeding with hemorrhagic shock should be avoided if possible. Knowledge of the risks and preparation for these problems is of great benefit here. For example, during mediastinoscopy, the risk of serious bleeding is low, less than 1% [7]. However, if there is a major loss of blood, the creation of 1 to 2 large-lumen venous accesses is extremely useful even before the start of the operation. For operations with a higher risk of bleeding, an appropriate number of red cell concentrates compatible for the recipient should be provided, depending on the hemoglobin level before surgery. A “patient blood management” concept is always recommended.
The incidence of acute respiratory failure after major thoracic surgery is reported in the literature to be between 2-7% [8]. Mortality from severe respiratory failure in the first 48 hours after pneumectomy ranges from 26% to 80%, depending on the literature. The main problem lies in the gas exchange, which is now limited to only one lung. If this function is impaired, the patient very quickly enters vital dangerous ranges of hypercapnia and hypoxia. It is therefore important to determine the volume supply precisely and often to limit the volume supply in quantity. The trauma of surgery, anesthesia, pain, etc., result in impaired lung function postoperatively. Bronchoconstriction, secretion, and aspiration favor the formation of atelectasis and pneumonia. Intraoperative ventilation during single-lung ventilation with reduced tidal volumes (5 ml/kgKG) and moderate PEEP (5 – 8 cm H2O) may improve postoperative gas exchange and reduce risks [9]. Early mobilization (from bed), respiratory and physical therapy, and good pain management (e.g., with a thoracic peridural catheter) improve the situation [10]. Noninvasive ventilation, bronchoscopic suctioning in spontaneous breathing, invasive ventilation with abdominal positioning if necessary, and tracheostomy in prolonged cases are necessary measures for treatment in cases of markedly impaired pulmonary gas exchange and atelectasis.
Risk of pneumonia increases with duration of ventilation
The risk of pneumonia increases proportionally to the duration of invasive ventilation [11]. For most patients after thoracic surgery, rapid restoration of spontaneous breathing and early extubation are reasonable. However, if pH-relevant hypercapnia, inadequate oxygenation, unstable circulation, or failure to adequately ventilate the affected lung segment after prolonged atelectasis of a lung, postoperative ventilation is appropriate. In patients with marked chronic obstructive pulmonary disease, it may sometimes be necessary to extubate despite elevated paCO2 levels (with protective reflexes present, adequate consciousness, and patient cooperation available) and to provide early support for respiratory mechanics with noninvasive ventilation. If a double lumen tube is used intraoperatively, it is recommended to change to a normal tube postoperatively to reduce the irritation caused by the endobronchial tube position and to achieve better secretion management. If there is or was a difficulty with orotracheal intubation, the use of a “Cook rod” (Airway Exchange Catheter) is recommended when changing the tube.
Positioning intubated patients with the upper body elevated by approximately 40° reduces the incidence of ventilator-associated pneumonia. Regular oral care and regular control of the cuff pressure are also useful. After the occurrence of pneumonia, microbiological sample determinations (from tracheal or bronchial secretions, always before starting antibiotic therapy) and sufficient antibiotic therapy are necessary. Gram-positive and Gram-negative pathogens must be expected; less frequently, fungi or atypical pathogens are the triggers of pneumonia. Antibiotic therapy may need to be adapted to the hospital-specific pathogen spectrum. Care must be taken to ensure adequate dosage and properly selected duration of therapy. In the vast majority of cases, a therapy duration of one week is sufficient. In particular, patients receiving immunosuppressive or neoadjuvant pretreatment have an increased risk of infection and are more difficult to treat. Often, patients with pneumonia also benefit from bronchospasmolysis with medication and systematic respiratory therapy.
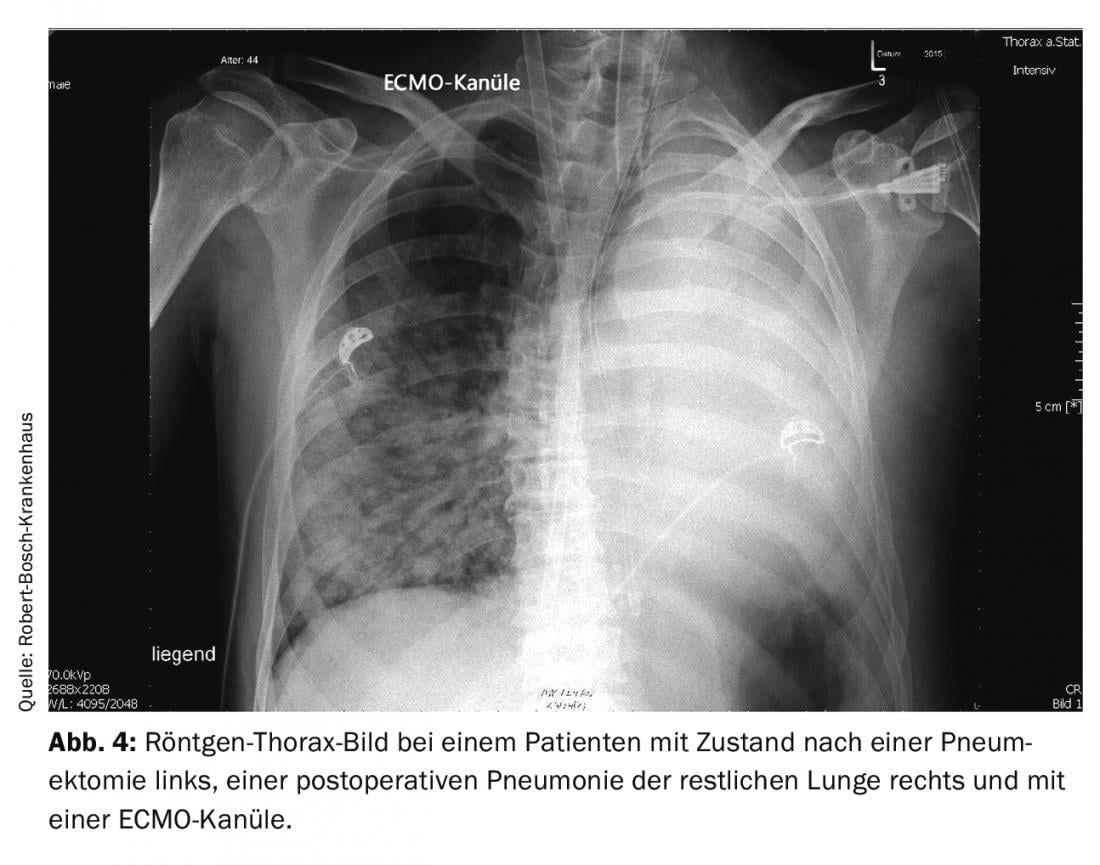
If all conventional measures fail in pulmonary failure, therapy with veno-venous extracorporeal membrane oxygenation (ECMO) may be necessary. In a patient with a post-pneumectomy condition, pneumonia in the remaining lung can rapidly become life-threatening due to the surgically induced reduction in gas exchange surface area. If the general conditions are right (no contraindications and a sufficiently stable patient in the other vital functions), ECMO therapy can bring significant advantages in such a course. Figure 4 shows an x-ray of a 44-year-old patient with pneumonia after pneumectomy and an ECMO cannula placed via the right internal jugular vein. In this case, therapy with ECMO was successfully completed after ten days. Since this extracorporeal procedure is not without risk, there must be a clear indication for this treatment and there should be sufficient experience with this procedure in the treatment team. The need for anticoagulation, injuries from placement of the large-lumen catheters, and nosocomial infection are the main risks of the procedure.
Re-expansion edema of the lung as another cause of postoperative gas exchange disturbance may occur after relief of a large pleural effusion or a pronounced pneumothorax and also after single-lung ventilation (lung separation via double lumen tube or bronchus blocker) over a long period of time. The pathophysiological cause is thought to be a multifactorial process. The development of fluid accumulation in the interstitium and alveoli due to increased fluid permeability is thought to involve the release of mediators, a loss of surfactant, and damage to the alveolar membrane by oxidases. Pathophysiologically similar is post-pneumectomy edema, this occurs 24 -72 hours postoperatively after pneumonectomy or rarely after lobectomy. Treatment consists of restrictive fluid intake, noninvasive or invasive ventilation with PEEP, and increased inspiratory oxygen concentration, usually required. It is associated with significant mortality, similar to severe ARDS, and unfortunately can occur even with very reduced fluid intake perioperatively.
Overall, close monitoring, rapid and correct recognition, and prompt treatment are necessary for the aforementioned vital threatening disorders. This makes it possible to achieve a good result for patients, even in procedures that are associated with a considerable perioperative risk.
Take-Home Messages
- Estimation of perioperative risk for mortality and lethality helps to avoid postoperative complications in major thoracic surgery. Systematic functional tests are helpful here.
- Respiratory and cardiac complications occur with relevant frequency during these procedures.
- Semi-invasive or invasive circulatory monitoring is recommended for unstable cardiovascular conditions; blood pressure and heart rate values alone are often insufficient.
- Mobilization, upper body elevation, respiratory and physical therapy, along with ventilator settings, are cornerstones of the prevention and treatment of respiratory complications. In most cases, early spontaneous breathing and rapid extubation are reasonable for these patients.
- In individual situations, ECMO therapy may be necessary postoperatively.
Literature:
- von Dossow V, Zwißler B: Structured patient handover in the perioperative phase – The SBAR concept. Anesth Intensivmed 2016; 57: 88-90.
- Kristensen SD, Knuuti J, Saraste A, et al: 2014 ESC/ESA Guidelines on non-cardiac surgery: cardiovascular assessment and management: The Joint Task Force on non-cardiac surgery: cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anaesthesiology (ESA). Eur J Anaesthesiol 2014; 31(10): 517-573.
- Zhang R, Kyriss T, Dippon J, et al: G. American Society of Anesthesiologists physical status facilitates risk stratification of elderly patients undergoing thoracoscopic lobectomy. Eur J Cardiothorac Surg 2018; 53(5): 973-979.
- Smulders SA, Smeenk FW, Janssen-Heijnen ML, et al: Actual and predicted postoperative changes in lung function after pneumonectomy: A retrospective analysis. Chest 2004; 125: 1735-1741.
- Zhao BC, Huang TY, Deng QW, et al: Prophylaxis against Atrial Fibrillation after General Thoracic Surgery: Trial Sequential Analysis and Network Meta-Analysis. Chest 2017; 151(1): 149-159.
- Hansen M: Hemodynamic monitoring – Advanced monitoring [Invasive and minimally invasive hemodynamic monitoring]. Anasthesiol Intensivmed Notfallmed Schmerzther 2016; 51(10): 616-625.
- Schirren M, Sponholz S, Oguhzan S, et al: Intraoperative bleeding during thoracic surgery: avoidance strategies and surgical treatment concepts]. Surgeon 2015; 86(5): 453-458.
- Kösek V, Wiebe K: Postoperative respiratory insufficiency and its treatment [Postoperative respiratory insufficiency and its treatment]. Surgeon 2015; 86(5): 437-443.
- Marret E, Cinotti R, Berard L, et al: Protective ventilation during anaesthesia reduces major postoperative complications after lung cancer surgery: A double-blind randomised controlled trial. Eur J Anaesthesiol 2018; 35(10): 727-735.
- Rogers LJ, Bleetman D, Messenger DE, et al: The impact of enhanced recovery after surgery (ERAS) protocol compliance on morbidity from resection for primary lung cancer. J Thorac Cardiovasc Surg 2018; 155(4): 1843-1852.
- Wunder C: Nosocomial infections in critical care. Anesth Intensivmed 2020; 61: 215-222.
InFo PNEUMOLOGY & ALLERGOLOGY 2021; 3(1): 9-13.