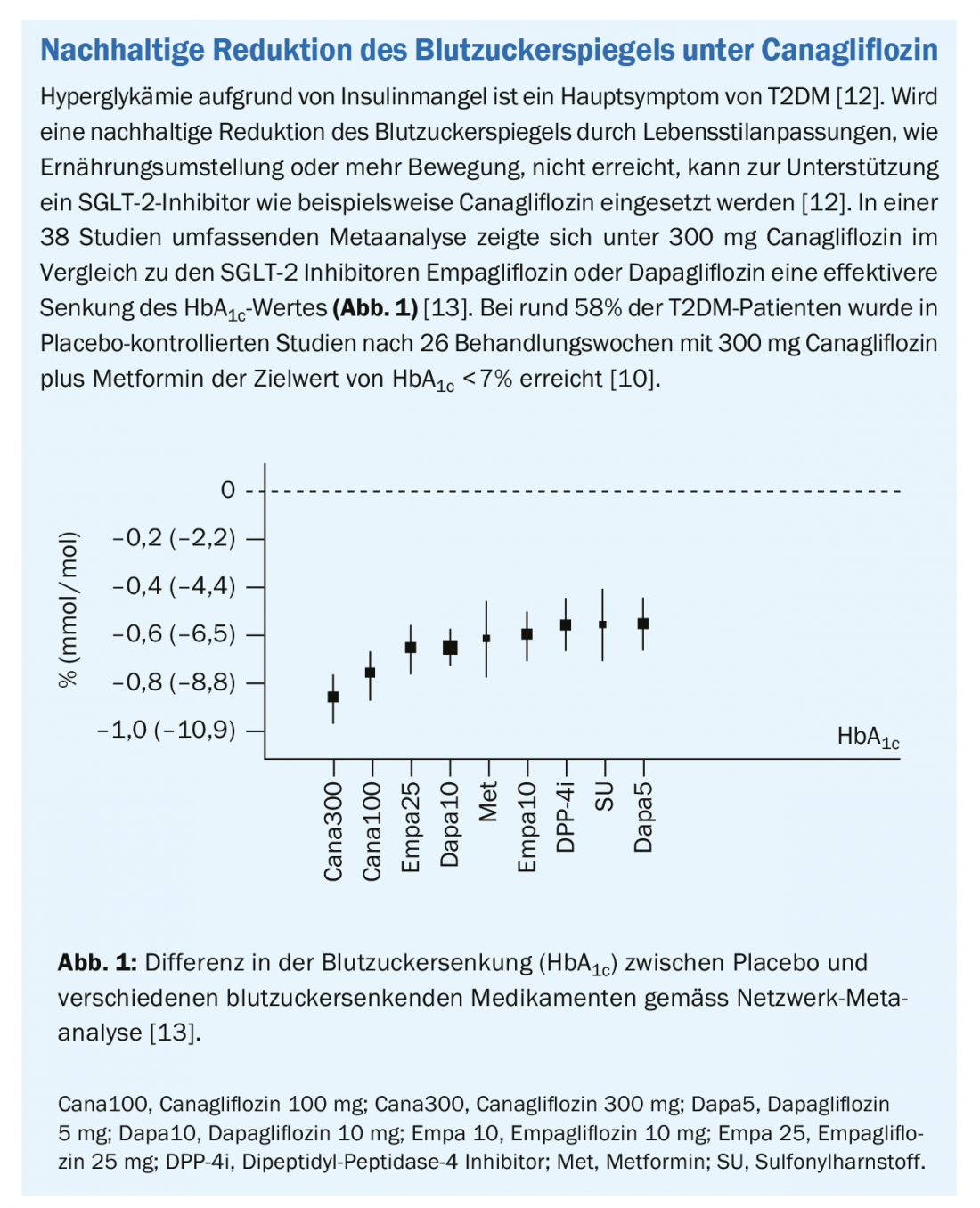
Type 2 diabetes mellitus (T2DM) is associated with a greatly increased risk of cardiovascular disease [1]. The joint management of T2DM and the cardiovascular risks has therefore come into focus [1].
Diabetes mellitus (DM) is one of the most common chronic diseases worldwide and the number of patients is continuously increasing [2,3]. In Switzerland, DM affects approximately 500,000 people, with 460,000 suffering from type 2 DM (T2DM) [3]. In this context, cardiovascular risk is also significantly increased [2]. According to the Emerging Risk Factor Collaboration meta-analysis, DM patients have a doubled risk of coronary heart disease, ischemic stroke, and vascular death independent of other risk factors [4]. Moreover, T2DM and cardiovascular disease are favored by the same typical risk factors, such as obesity, hypertension, insulin resistance, and dyslipidemia [2]. Therefore, cardiovascular risk reduction in the context of T2DM therapy has become increasingly important in recent years [5].
Cardiovascular risk reduction in focus
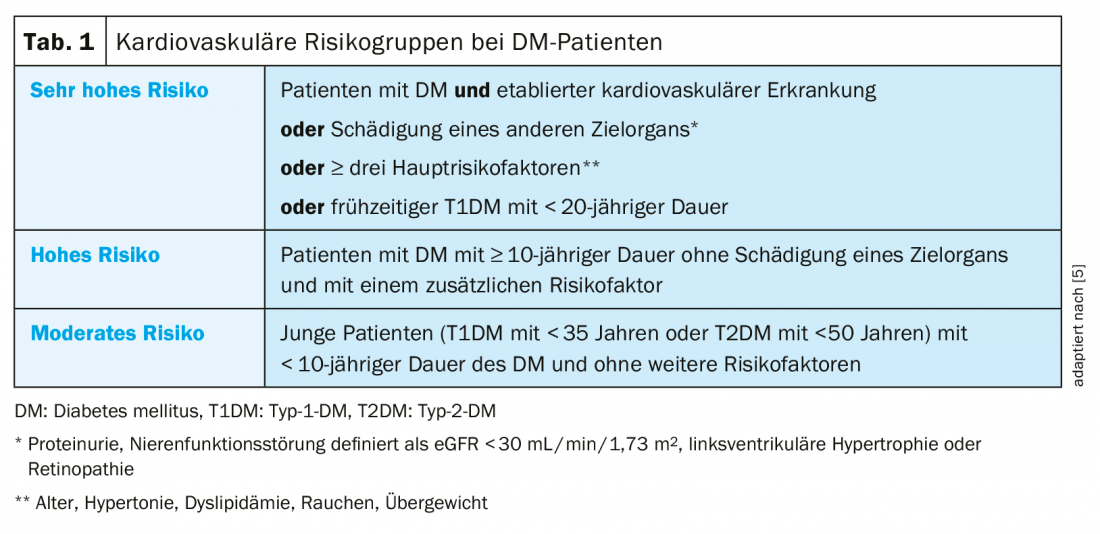
The management and prevention of cardiovascular disease associated with T2DM are the focus of the 2019 updated European Society of Cardiology (ESC) and European Association for the Study of Diabetes (EASD) guidelines [5]. For this purpose, new cardiovascular risk categories (moderate, high, very high risk) were defined, which also take into account the duration of DM and comorbidities (Table 1) [5]. In addition, metformin is now provided in the first line of treatment only in overweight patients with no, or moderate, cardiovascular risk. In patients at high or very high cardiovascular risk, treatment with an SGLT-2 inhibitor or GLP-1 receptor agonist is preferred in the first line of therapy instead of metformin [5]. The use of an SGLT2 inhibitor or GLP-1 receptor agonist in combination with metformin as early as possible is also recommended in the new guidelines of the Swiss Society of Endocrinology and Diabetology (SGED) [6].
Improved cardiovascular outcome with canagliflozin.
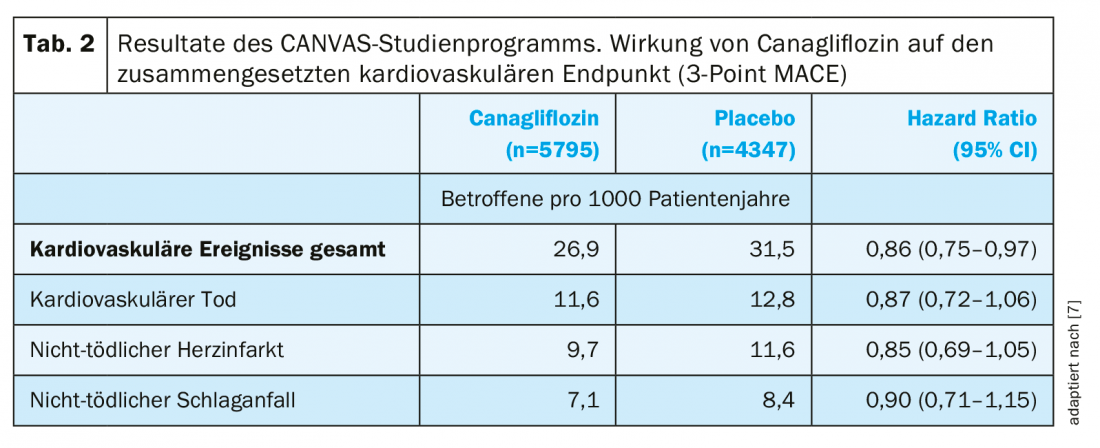
The two sister studies of the CANVAS program enrolled more than 10,000 patients with T2DM and high to very high cardiovascular risk and randomized them to treatment with canagliflozin or placebo [7]. The primary endpoint 3-point MACE, consisting of cardiovascular death, nonfatal myocardial infarction, and nonfatal stroke, was observed significantly less frequently with canagliflozin compared with placebo (superiority, p=0.02, Tab. 2) [7]. Furthermore, except in patients on diuretics, the benefit of canagliflozin therapy was achieved in all defined subgroups [7]. The majority of the adverse effects observed with canagliflozin corresponded to the already known safety profile [7]. Overall, serious adverse events occurred less frequently with canagliflozin compared with placebo (104.3 vs. 120.0 affected per 1000 patient-years, HR: 0.93) [7]. The risk of amputation was increased with canagliflozin (6.3 vs. 3.4 affected per 1000 patient-years; HR, 1.97), but no causal relationships between canagliflozin therapy and the increased risk of amputation could be established. Most amputations occurred at the level of the toe or midfoot, and risk factors included a history of amputation, peripheral arterial disease, or neuropathy [7,8]. In another study, no increased risk of amputation was observed with canagliflozin [9].
Indication expansion of canagliflozin
Canagliflozin has been approved since 2014 as an add-on therapy to diet and exercise for the treatment of adult patients with inadequately controlled T2DM or in combination with other antihyperglycemic medications [10]. Based on the data from the CANVAS trial program, which demonstrated the cardioprotective effect of canagliflozin in patients with T2DM and high cardiovascular risk, the indication expansion for canagliflozin (Invokana®) and the fixed combination of canagliflozin and metformin (Vokanamet®) for the prevention of cardiovascular events in adults with T2DM and already manifest cardiovascular disease was recently made [10,11].
Conclusion
Canagliflozin therapy significantly reduced cardiovascular events in patients with T2DM at high cardiovascular risk compared with placebo in the CANVAS trial program [7]. The registration extension for canagliflozin based on these data now opens a new therapeutic option for the prevention of cardiovascular events in patients with T2DM and already manifest cardiovascular disease [10].
Literature:
- Einarson TR, et al: Prevalence of cardiovascular disease in type 2 diabetes: a systematic literature review of scientific evidence from across the world in 2007-2017. Cardiovasc Diabetol, 2018. 17(1): p. 83.
- De Rosa S, et al: Type 2 Diabetes Mellitus and Cardiovascular Disease: Genetic and Epigenetic Links. Front Endocrinol, 2018. 9: 2.
- Website of Diabetes Switzerland “About Diabetes”. https://www.diabetesschweiz.ch/ueber-diabetes.html. Last access: 20.02.2020.
- Sarwar N, et al: Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet, 2010. 375(9733): 2215-2222.
- Cosentino F, et al: 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J, 2020. 41(2): 255-323.
- Swiss Society of Endocrinology and Diabetology (SGED/SSED) recommendations for the management of type 2 diabetes mellitus (2020). https://www.sgedssed.ch/fileadmin/user_upload/6_Diabetologie/61_Empfehlungen_Facharzt/2020_Swiss_Recomm_Medis_DE_def.pdf. Last access: 25.03.2020.
- Neal B, et al: Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N Engl J Med, 2017. 377(7): 644-657.
- Matthews DR, et al: Effects of canagliflozin on amputation risk in type 2 diabetes: the CANVAS Program. Diabetologia, 2019. 62(6): 926-938.
- Perkovic, V., et al, Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N Engl J Med, 2019. 380(24): 2295-2306.
- Specialty Information Invokana®. www.swissmedicinfo.ch. State of Information: October 2019.
- Specialty Information Vokanamet®. www.swissmedicinfo.ch. State of Information: October 2019.
- Wilding JP, et al: Efficacy and safety of canagliflozin by baseline HbA1c and known duration of type 2 diabetes mellitus. J Diabetes Complications, 2015. 29(3): 438-444.
- Zaccardi F, et al: Efficacy and safety of sodium-glucose co-transporter-2 inhibitors in type 2 diabetes mellitus: systematic review and network meta-analysis. Diabetes Obes Metab, 2016. 18(8): 783-794.
Responsible for content and financed by Mundipharma Medical Company, Basel Branch.
PR202005