A confirmed diagnosis, patients who are convinced of the sense and purpose of the treatment, this may sound banal, but with regard to a possibly lifelong drug treatment, these aspects are highly relevant. In addition to a concise overview of various active ingredients, special features of drug treatment in children will be addressed.
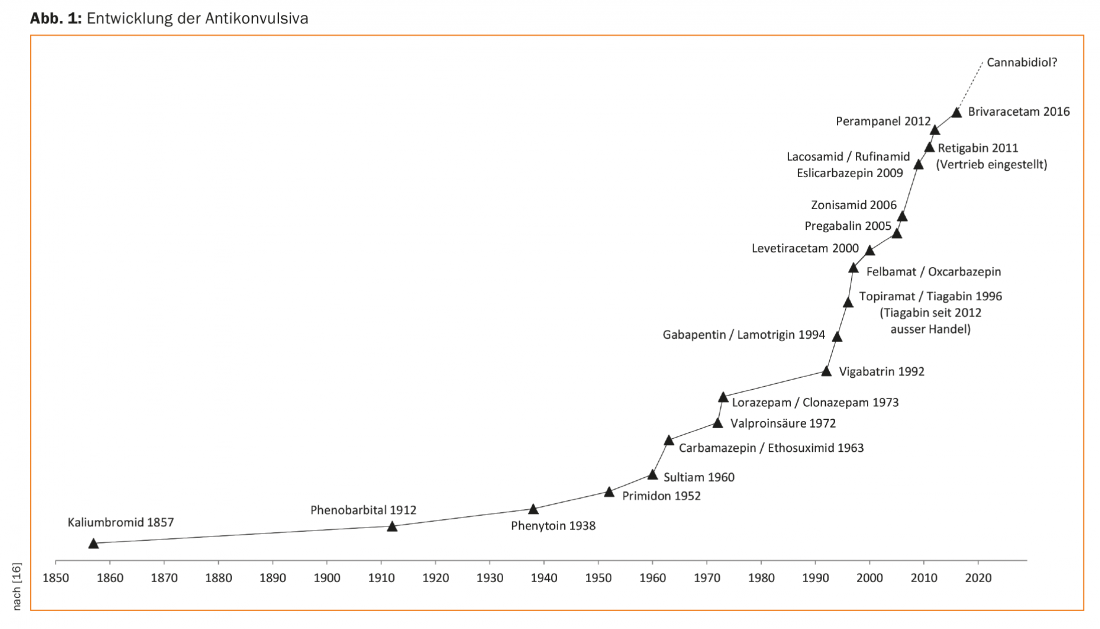
Epileptic seizures and epilepsies are common clinical situations and have been known for millennia. Already on cuneiform tablets from the time of the Babylonians from the 2nd Jtsd. BC first medical texts on epilepsies are recorded. Over the centuries, therefore, a correspondingly large number and variety of procedures have been applied to epileptic seizures. Although already Hippocrates around 500 BC. Epilepsy was considered to be a disease originating in the brain, but this knowledge was forgotten and so for a long time (and still today in some regions of the world) patients with epilepsies were considered to be possessed by demons and treated with religiously based exorcisms and other measures. Only in the 17th/18th century and especially then in the 19th century, the present scientific view slowly prevailed [1]. Queen Victoria’s gynecologist, Sir Charles Locock, first described the antiepileptic effects of potassium bromide in Lancet in 1857, establishing the modern drug treatment of epilepsies [2]. In 1912, another drug was added, phenobarbital, which still plays a role in the treatment of epilepsy today. Since then, a number of substances have been discovered or (Fig. 1), although none of these substances has yet been shown to have an actual antiepileptic effect, only an anticonvulsant, i.e., seizure-suppressing, effect. It would therefore be more correct to speak of anticonvulsants and not of antiepileptics.
In order to be able to carry out a good drug therapy for epilepsy, the diagnosis of epilepsy must first of all be confirmed. This may sound banal, but it is by no means so in everyday clinical practice, since often, especially at the beginning of epilepsy, only rudimentary seizure descriptions are available and the rate of misdiagnosis is astonishingly high. In particular, syncope, due to e.g. cardiac arrhythmias or circulatory dysregulation, may also be accompanied by clonic discharges, thus feigning a tonic-clonic epileptic seizure. Functional disorders or hyperkinetic movement disorders such as myoclonias should also be considered in the differential diagnosis. Since the treatment of epilepsy, especially in adults, often results in lifelong drug therapy and requires a high level of therapy adherence, the patient must be convinced of the purpose of the treatment. Therefore, in cases of uncertainty, a long-term video EEG examination should be considered at a low threshold to confirm the diagnosis.
Causes
In addition to history and seizure description, EEG and imaging, preferably cMRI, are primarily used to diagnose epilepsies. The technical progress of MR devices should also be taken into account here, so that if MRI is negative, repeating it with a newer device and performing it with specific epilepsy MR protocols may be helpful. The aim of diagnostics is to clarify the cause or cause of the disease as accurately as possible. classification into an epilepsy syndrome can be achieved, which influences the selection of the appropriate anticonvulsant.
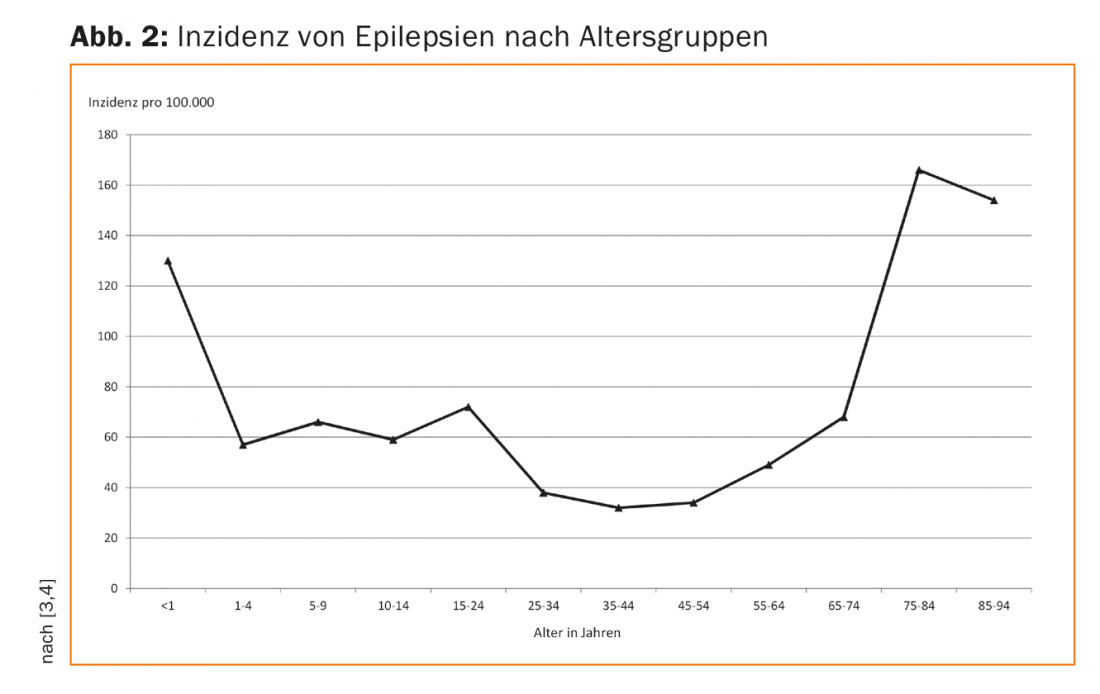
Naturally, the causes of epilepsy in children and in adults differ to some extent. Thus, in children, epilepsies due to genetic predispositions (as in absence epilepsy and Rolando’s epilepsy), due to congenital malformations of the brain or perinatal asphyxia, and seizures due to metabolic disorders (e.g., the vitamin B6-dependent epilepsies) predominate. Epilepsies due to acquired brain injury also occur in children. However, in contrast to children, these represent the vast majority of new-onset epilepsies in adults. Due to demographic change, the incidence of epilepsy in the elderly is increasing significantly, leading to new challenges in drug therapy in this age group (Fig. 2).
Pediatric Epileptology
In the context of the diagnosis and therapy of epilepsies in children, the developmental stage and age of the children are added as further dimensions. Thus, the frequency and type of epilepsies differ markedly in the respective pediatric age groups. Historically, it has long been unclear how to classify the many syndromes within the large group of seizure disorders; here, the development of EEG diagnostics by Hans Berger in the middle of the last century brought great progress. Examples include Watanabe epilepsy, West syndrome, Rolando epilepsy, and absences epilepsy. Watanabe epilepsy, for example, is the benign seizures of the newborn that occur in the first three months and disappear by the end of the first year of life. West’s syndrome was first described by William James West in Tonbridge in 1841, depicting the characteristic flash Nick Salam seizures. West syndrome is a generalized epilepsy that is associated with spasms and typically occurs between three and six months of age. It is triggered by a variety of possible causes (polyetiology). In this regard, West syndrome is a good example of how different causes lead to a common pathophysiological final pathway, presenting the same electroencephalographic and semiological clinical picture. Another classic pediatric seizure disorder is Rolando’s epilepsy, benign idiopathic focal epilepsy. This also has a typical age and most often occurs in toddlers and young school children. Characteristic is the onset with tingling paresthesias and motor discharges in the area of the face and not infrequently with speech arrest. The EEG shows classic triphasic “sharp-waves” over the central region. In the slightly older children, one observes the classic juvenile absencenepilepsy, which is associated with rigidity and lack of responsiveness to response. Often the suspected diagnosis is made by teachers because the children seem absent in class due to “daydreaming”. The EEG shows generalized 3/s spike-wave paroxysms during the absences.
From this excerpt of pediatric epilepsy syndromes it can already be guessed that therapy should be specific according to characterization of the epilepsy syndrome [5].
Drug therapy in children
The choice of anticonvulsant for continuous therapy in children is crucially shaped by the characterization of the seizure disorder. In addition to the epilepsy syndrome, other aspects such as the development and the therapeutic goal are of course decisive for the choice. However, for all epilepsies, acute seizure therapy relies on a benzodiazepine, usually midazolam or clonazepam. Oral, rectal, and nasal dosage forms of midazolam are available for the outpatient setting.
In Watanabe epilepsies and benign infantile epilepsies, carbamazepine or oxcarbazepine have become widely used. West syndrome is classically treated with a regimen that includes vigabatrin and steroids. Rolando’s epilepsy is classically treated primarily with sultiam or oxcarbazepine, and absences epilepsy is usually treated with ethosuximide.
In addition to these classic epilepsy syndromes, there are of course also epilepsies in children that are the result of structural changes, e.g. after an accident or an infection. Here, lamotrigine, levetiracetam, or oxcarbazepine have become established, similar to those used in adults. In many other epilepsy syndromes, such as juvenile myoclonic epilepsy or Lennox-Gastaut syndrome, as well as myoclonic-astatic epilepsy, valproate has been established as the first-line agent [7].
Generally, in pediatric epileptology, monotherapy should be sought whenever possible to avoid negative impact on child development [6]. When considering treatment goals, it is important to keep in mind that the child’s mobility level has a critical impact on the treatment goal. For example, a child who moves around in traffic or goes to the swimming pool is significantly more at risk for seizures than an immobile child. With increasing age and independence from parents, other aspects such as taking medication independently and therapy compliance also come to the fore.
In many epilepsy syndromes, a not insignificant number of patients will be seizure-free even without medication after completion of brain maturation, so the pediatric epileptologist should plan an omission trial. It should therefore be discussed with the family at an early stage when and how a remission attempt could be made, even if this is still several years in the future. In addition, for patients who will be dependent on anticonvulsants for the rest of their lives, care must be taken to ensure continuity of care during transition from pediatrics to adult medicine.
Drug therapy for adults
Once the diagnosis of epilepsy has been confirmed, it is necessary to discuss with the patient which target symptom should be treated. In most cases, these will be the “large”, bilaterally spread tonic-clonic seizures (“grand mal”), which represent a considerable burden for the affected person, but of course also for the relatives, and carry a risk of injury up to sudden unexpected death (SUDEP – “sudden unexpected death in epilepsy patients”). Even purely focal seizures until then can occasionally lead to a large bilaterally spread tonic-clonic seizure with corresponding consequences. Some patients notice resp. do not remember their seizures, which is why the term “seizure-free” is often difficult to define in clinical practice. Repeated epileptic seizures resp. a high seizure frequency (including subclinical seizures) can also lead to cognitive impairment in the course [8]. The indication for treatment must therefore be discussed with the patient in the given individual case.
Treatment then begins with an appropriate anticonvulsant (see below), which is dosed up to an initial target dose. If further seizures occur under this dose, the dose of this preparation should first be exhausted to the tolerance threshold before a switch to another substance or combination therapy (add-on) is established. The tolerance limit varies greatly depending on the substance and the individual.
A number of substances are now available for the treatment of epilepsies in adults. The most commonly used agents today are lamotrigine, levetiracetam, valproate, and carbamazepine, with new preparations coming onto the market all the time. However, the newer agents are not necessarily more potent compared to the older agents, but they have significantly fewer side effects and usually fewer interactions with other drugs [7]. Nevertheless, usually in the large drug trials, which are mostly conducted in refractory epilepsy patients, a certain proportion of patients (about 10-20%) even become seizure-free with a new substance, so that a corresponding therapy trial with a new substance may well be justified.
Lamotrigine: This drug has been available since the mid-1990s and is usually very well tolerated with no side effects. It can be used for both focal (structural) and generalized epilepsies. It is an oral sodium channel blocker only. The main disadvantage is that it must be dosed very slowly to avoid the dreaded allergic skin reactions, which can go as far as life-threatening Lyell’s or Stevens-Johnson syndrome. The usual first target dose is 150 mg (in the elderly) and 200 mg (standard dose) and is reached only after 4-6 weeks. Because of its long half-life of about 24-35 hours, lamotrigine may be given only once a day. If necessary, the dose can often be significantly increased to up to 500-800 mg per day during the course due to good tolerability [9].
Use during pregnancy is possible and associated only with a slightly increased risk of malformation [10]. In everyday life, some relevant drug interactions of lamotrigine should be noted: the ethinylestradiol in hormonal contraceptives with estrogen and progestin components (“the pill”) can practically halve the plasma level of lamotrigine, which on the one hand can lead to insufficient seizure protection while taking the pill and on the other hand to significantly increased levels during the pill break with corresponding side effects [11]. Women must therefore be counseled appropriately, and we recommend either a progestin-only preparation or an IUD for lamotrigine therapy. In the case of combination therapies, the interaction with valproate in particular must be taken into account. Valproate significantly inhibits the breakdown of lamotrigine, so a much slower up-dosing regimen and a lower total dose of lamotrigine must be selected [9]. On the other hand, this combination is often very successful because of the slow metabolization and therefore only minor fluctuations in levels.
Levetiracetam: This drug can be used for focal as well as generalized epilepsies and is available as tablets, syrup, and for intravenous use, so it plays an important role in acute hospital therapy. The initial target dose is 750 mg (elderly) to 1000 mg (standard dose). It can be increased to approximately 4000 mg and is given twice daily (half-life approximately 7 hours). The effect is mediated via the pre-synaptic vesicle protein SV2. Levetiracetam is poorly bound to plasma proteins and has virtually no interaction potential. However, psychiatric symptoms such as irritability, inner restlessness, aggressiveness, anxiety and depression may occur as major side effects. In the literature, the rate of these side effects is reported to be 10-15%; in clinical practice, although not always leading to a change in therapy, this rate seems to be even higher, at 20-30%. Therefore, its use in patients with behavioral problems and/or frontal brain lesions, for example, is not favorable. Levetiracetam has a good effect on myoclonia. Together with lamotrigine, it is the drug of first choice in pregnancy.
A further development of levetiracetam is brivaracetam (Briviact®), which was approved as the newest anticonvulsant in Switzerland at the end of 2016. This drug has fewer psychiatric side effects than levetiracetam with (at least) equal efficacy, target dose is 100-200 mg divided into two doses, it can be dosed quickly resp. the target dose can be started directly. When switching from levetiracetam to brivaracetam (e.g., because of psychiatric symptoms), an “overnight” switch can be made at a ratio of 10-15 to 1 (e.g., 1000 mg levetiracetam to 100 mg brivaracetam).
Valproate: Valproate is a substance discovered in the 1960s that continues to play an important role in the treatment of epilepsy. It likely acts by enhancing GABAergic mechanisms and is used in focal epilepsies as well as generalized epilepsies. Especially for the latter, it is often considered the most effective therapy. The usual initial target dose is 1000 mg divided into two doses, although the sustained-release preparation may be given only once daily. Unlike most other anticonvulsants, valproate is predominantly hepatically metabolized, so it can be used in renal insufficiency. The most relevant side effects are often significant weight gain and possible cognitive slowing. Valproate is a potent enzyme inhibitor, so relevant interactions must be considered during treatment in clinical practice. When combined with lamotrigine, the degradation of lamotrigine is inhibited, which significantly increases lamotrigine levels, which may lead to signs of intoxication (see above). Antibiotic therapy with carbapenem may result in a substantial drop in valproate levels within 24 to 48 hours, which may extend to the induction of status epilepticus [12]. Metabolism in the liver may result in increased ammonia production, which may cause encephalopathy. For women of childbearing age, resp. it must not be used because of the significant teratogenicity resp. only be used after a detailed risk-benefit assessment and a written declaration of consent by the woman concerned (see also section Pregnancy).
Carbamazepine: Carbamazepine was also discovered as early as the 1960s. It is a sodium channel blocker used for focal epilepsies. In generalized epilepsies, there may be worsening of the seizure situation, especially myoclonias and absences. The usual first target dose is 800 mg divided into two doses (half-life due to auto-induction 16-24 hours, with single dose approximately 36 hours [9]). The most relevant side effect of carbamazepine is dizziness, sometimes with nystagmus, due to its narrow therapeutic range. Skin reactions are also not uncommon with carbamazepine, which is associated with specific HLA alleles present primarily in Japanese, Chinese, and Thai, but somewhat less commonly in Caucasians. Because carbamazepine is a potent enzyme inducer and therefore many drug interactions exist, its use in elderly patients is often limited, although there is good anticonvulsant activity per se. It can be used in pregnancy, although miscarriage rates are somewhat higher than with lamotrigine and levetiracetam [10].
Special situations – Pregnancy
There is a particular need for counseling in young women of childbearing age with epilepsy with regard to a possible pregnancy. Epilepsy per se is not a reason not to get pregnant and in over 90% women with epilepsy usually give birth to healthy children without complications. However, there are some important things to keep in mind [13]. Folic acid substitution should already be started if a child is desired, i.e. before pregnancy occurs (see also the article on epilepsy and the desire to have children on page 18 in this issue).
The most important thing is certainly the optimal medication setting to protect the woman and the unborn baby from seizures during pregnancy. The first choice in pregnant women is lamotrigine and levetiracetam, which showed only a small increased risk of malformation in large prospective registry studies (e.g., EURAP registry) [10]. In contrast to lamotrigine and levetiracetam, the risk of malformation with valproate is very high (dose-dependent up to approx. 45%(!), especially neural tube defects, but also cognitive impairment of the child [14]). The teratogenic effect exists primarily in the first weeks of pregnancy, when the woman often does not even know she is pregnant, so that valproate should not be used in women of childbearing age if possible; corresponding warnings (“red hand letter”) have been issued by the regulatory authorities. It would be desirable to include all women with epilepsies and therapy with anticonvulsants in a pregnancy registry such as the EURAP registry mentioned above to obtain even more reliable information on the teratogenicity of the individual substances. The corresponding forms can be downloaded from the Internet and filled out by any physician and sent to an appropriate study center.
Limits of therapy – epilepsy surgery
Although freedom from seizures is often the primary goal of treatment, this is achieved on average in only about 60-70% of patients, depending on the epilepsy syndrome. Even with combination therapies, only a few patients become seizure-free in addition. These rates have not increased significantly in recent decades, even with newer medications. Therefore, in addition to verifying the diagnosis of epilepsy and compliance, epilepsy surgery should be considered early. This can lead to permanent recovery from epilepsy in suitable patients with focal epilepsies in up to approximately 80-90%, depending on the seizure origin. In recent years, therefore, a rethinking has begun here away from epilepsy surgery as the last resort to a therapy of (almost) first choice (e.g., in mesial temporal lobe epilepsy in hippocampal sclerosis – see also the article the article on surgical treatment on page 26 in this issue. [15]).
While in some childhood and adolescent epilepsies the disease is known to “heal”, this is rarely the case in adulthood. Nevertheless, a cautious discontinuation attempt may be warranted in some circumstances (DD misdiagnosis in the past?). On the other hand, if the cause of epilepsy persists (e.g., malformation, posttraumatic or postischemic defect) and seizure freedom is present, it should not be jeopardized.
Pragmatically, we dose down to a low dose in generalized epilepsies, long-standing seizure freedom, and explicit patient request, while performing EEG monitoring (EEG is better at predicting seizure recurrence risk in generalized epilepsies than in focal epilepsies). We then carry out the final abdosage step under long-term video EEG monitoring, but of course there remains a certain residual risk of a relapse seizure, which the patient must be prepared to bear.
Take-Home Messages
- The diagnosis of epilepsy must be confirmed.
- The frequency and types of epilepsies differ markedly among pediatric age groups.
- The choice of antiepileptic drug for long-term therapy in children is crucially shaped by the characterization of the seizure disorder.
- In many epilepsy syndromes, patients will be seizure-free without medication once brain maturation is complete, so an attempt at cessation should also be planned for the long term.
- In pediatric epileptology, monotherapy should be pursued whenever possible.
- Dosages should be increased from the first target dose, depending on seizures, up to the tolerance limit; only then switch to other preparation or add-on (combination therapy/in adults).
- Drug interactions should be noted: Carbamazepine is an enzyme inducer, valproate is an enzyme inhibitor, carbapenems can dramatically decrease valproate levels.
- Valproate should not be used in women of childbearing age if possible.
Literature:
- Gonzalo A (ed.): Introduction to Epilepsy. Cambridge University Press; 2012.
- Brody MJ: Antiepileptic drug therapy – the story so far. Seizure 2010 Dec; 19(10): 650-655.
- Werhahn KJ: Epilepsy in the elderly. Dtsch Arztebl Int. 2009; 106(9): 135-142.
- Olafsson E, et al: Incidence of unprovoked seizures and epilepsy in Iceland and assessment of the epilepsy syndrome classification: a prospective study. Lancet Neurol 2005; 4: 627-634.
- Broser P, Maier O: Early infantile epileptic encephalopathies. Epileptology 2016; 33: 95-101.
- Pellock JM, Bourgeois BFD, Dodson WE (eds.): Pediatric Epilepsy. New York, Demos, 2007.
- Steinhoff B, Bast T: Vademecum Antiepilepticum. German Society for Epileptology 2017.
- Vingerhoets G: Cognitive effects of seizures. Seizure 2006; 15(4): 221-226.
- Swissmedic: www.compendium.ch. Retrieved July 2018
- Tomson T, et al: Comparative risk of major congenital malformations with eight different antiepileptic drugs: a prospective cohort study of the EURAP registry. Lancet Neurol 2018 Jun; 17(6): 530-538.
- Sabers A, et al: Lamotrigine plasma levels reduced by oral contraceptives. Epilepsy Res 2001 Nov; 47(1-2): 151-144.
- Sutter R, Rüegg S, Tschudin-Sutter S: Seizures as adverse events of antibiotic drugs: A systematic review. Neurology 2015 Oct 13; 85(15): 1332-13341.
- Voinescu PE, Penell PB: Management of epilepsy during pregnancy. Expert Rev. Neurother 2015; 15(10): 1171-1187.
- Meador KJ et al: Fetal antiepileptic drug exposure and cognitive outcomes at age 6 years (NEAD study): a prospective observational study. Lancet Neurol 2013 Mar; 12(3): 244-252.
- Steinhoff BJ, Staack AM: Is there a place for surgical treatment of nonpharmacoresistant epilepsy? Epilepsy Behav 2018.
- Golyala A, Kwan P: Drug development for refractory epilepsy: The past 25 years and beyond; Seizure 2017 Jan; 44: 147-156.
InFo NEUROLOGY & PSYCHIATRY 2018; 16(5): 12-17.