Acute lymphoblastic leukemia is the most common childhood cancer. It is treated in a risk-adapted manner and is curable in the majority of cases. New innovative drugs such as immunotherapies are in clinical trials.
Acute lymphoblastic leukemia (ALL) is the most common childhood cancer, accounting for approximately 30% and 3.3 new cases per 100,000 population under age 15. The age peak is around five years. In Switzerland, approximately 50-60 children are diagnosed with ALL each year. The most immunologically common subtype in childhood is B-precursor ALL, which develops from immature cells of the B-series of the lymphatic system. More rarely, ALL of the T lymphopoiesis occurs. A special form is mature B-cell leukemia, which is due to malignant transformation of the mature B-cell and is understood as a leukemic manifestation of Burkitt’s lymphoma. ALL is a heterogeneous disease characterized by uncontrolled proliferation of lymphoid progenitor cells in the bone marrow and peripheral blood [1]. It is now considered to be a disease that often has great similarities morphologically but can have very heterogeneous subentities cytogenetically or molecularly [2], which also correlates with a heterogeneous clinical response to treatment. Modern sequencing techniques can demonstrate the enormous clonal heterogeneity of this disease.
Causes
The cause of the development of leukemia remains unclear. Known but rarely occurring factors are ionizing radiation and congenital syndromes. However, this explains less than 10% of all diseases. Children with Down syndrome have about a 20-fold higher risk of developing leukemia (ALL or acute myeloblastic leukemia) within the first five years of life compared to unaffected, healthy children. Even more frequently (in 3-10%), however, transient myeloproliferation occurs in the neonatal period in these children, which occasionally may later progress to leukemia. Other rare congenital changes with increased risk of leukemia include ataxia teleangiectatica, Fanconi syndrome, and other syndromes associated with immunodeficiency or increased chromosomal fragility. The fact that ALL occurs in clusters between the second and fifth year of life, a clustering of the disease in industrialized countries, and the observation that clustering has occurred repeatedly in the past, especially in regions of new agglomerations, led to various infection-associated hypotheses of leukemogenesis [3,4].
Symptoms
The proliferation of leukemic blasts in the bone marrow leads to displacement of normal hematopoiesis, which explains the most common symptoms such as pallor and fatigue due to anemia or bleeding tendency due to thrombocytopenias. Infiltration leads to diffuse bone pain and alternating arthropathies, which occasionally manifest themselves in young children as reluctance to move or even refusal to walk. Furthermore, lymph node swelling as well as organomegaly may occur.
Diagnostics
In the blood, changes in at least two blood cell series are often found, most commonly thrombocytopenia with concomitant anemia. The leukocyte count may be normal, decreased or increased. The morphology of the blood count provides important diagnostic clues, and the final diagnosis is made by bone marrow aspiration. This is used to determine the immunophenotype of the leukemic blasts by flow cytometry (FACS) in addition to examining the morphology, and to perform chromosomal analysis. Immunophenotyping allows the determination of the developmental stage of the corresponding cell clone. The most common leukemia subtype in children, so-called “common-ALL”, is characterized by expression of the B-cell markers CD10 and CD19. Expression of myeloid antigens, though usually not prognostically significant, can be detected in up to half of ALL. Nowadays, cytogenetic and molecular genetic examinations are becoming increasingly important. It is important to identify the most important subgroups, as they have therapeutic implications. On the one hand, both numerical chromosomal alterations such as hyperdiploidy or hypodiploidy and structural alterations such as translocations, e.g. t(12;21) (fusion of ETV6/RUNX1 genes) or t(9;22) (fusion of BCR/ABL1), MLL rearrangements (MLL 11q23) and other alterations are sought.
Classically, detection of these alterations is performed with conventional cytogenetics (G-banding) and/or fluorescent in situ hybridization (FISH) in leukemia cells. In recent years, the measurement of minimal residual disease (MRD) from bone marrow has become established in the context of follow-up diagnostics to assess the response of leukemia to treatment. Treatment response has now emerged as one of the most important prognostic parameters. Today, two main methods are used for progression diagnostics, which complement each other in clinical practice. The most sensitive method is monitoring immunoglobulin and T-cell receptor rearrangements. This involves an initial search for leukemia-specific clonal rearrangements that are followed by quantitative PCR at specific therapy time points. The detection limit thus achieved is approximately one leukemia cell per 100,000 normal bone marrow cells. A technique of MRD measurement that is less sensitive by approximately one log level is based on monitoring of the leukemia-associated immunophenotype by FACS. A sensitivity of 0.001% can be achieved [5].
Treatment
As recently as the 1970s, less than 30% of children survived the disease, whereas today nearly 85% of patients can be cured in the long term (Fig. 1) . Progress in survival rates over the last ten to 15 years has been achieved mainly through better risk stratification and consequent risk-adapted treatment. Current ALL frontline therapy essentially consists of the combination of corticosteroids, amino acid or substrate depletion (asparaginase, methotrexate), alkylating agents, antimetabolites, classical metaphase blockers, and anthracyclines [6]. Newer agents, so-called “targeted” therapeutics, have had very limited use in the treatment of pediatric ALL, with a few exceptions such as tyrosine kinase inhibitors in Philadelphia chromosome-positive ALL. Recently, new rare subgroups of B-precursor ALL have been identified, so-called “Philadelphia like” (or “BCR-ABL like”), which on the one hand show a significantly increased risk of recurrence, but on the other hand might be potential candidates for targeted therapies [7]. However, most of the new high-risk genetic criteria have also been shown to have variable impact, depending on the measurable response to therapy.
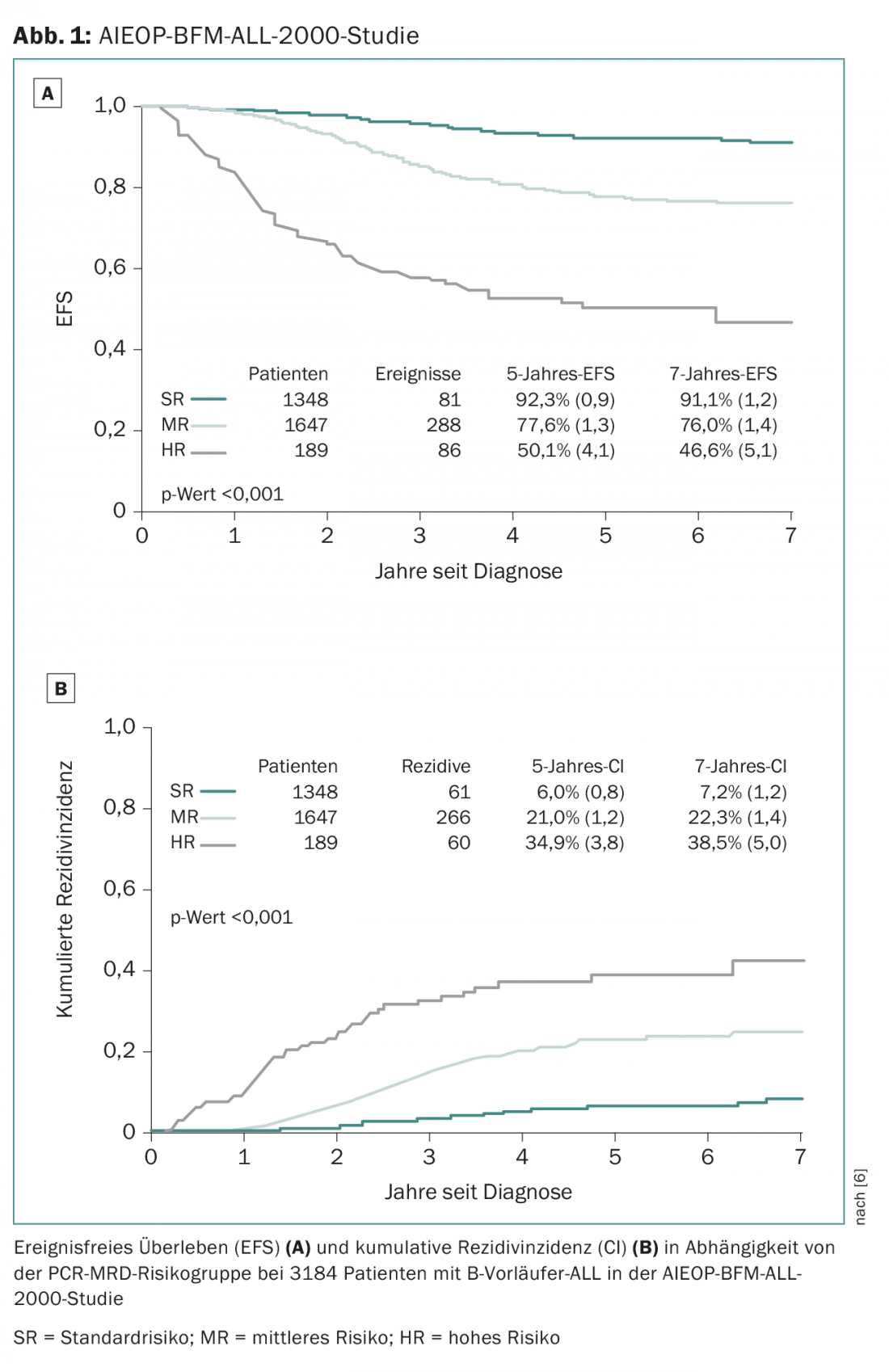
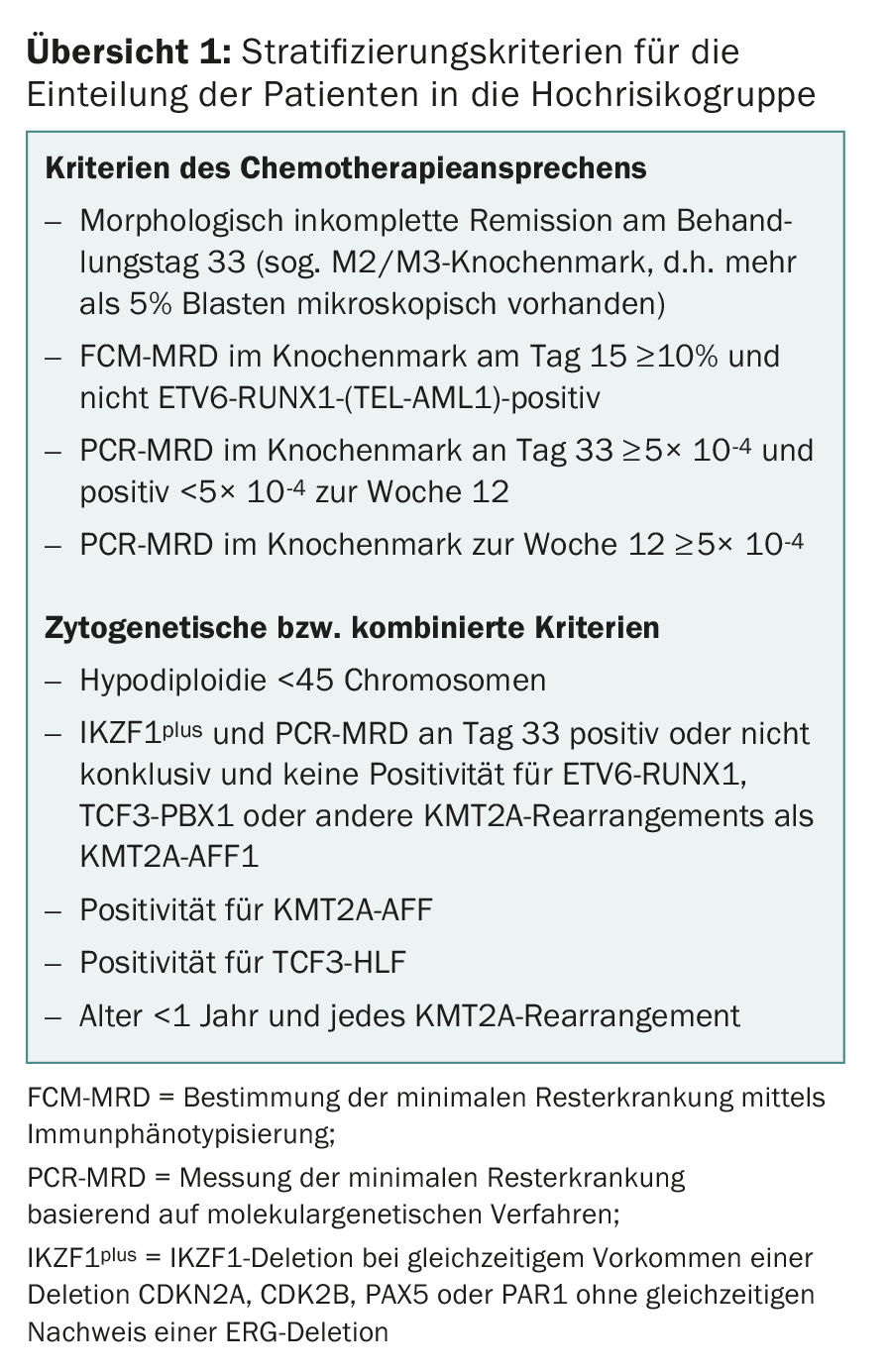
Most Swiss pediatric oncology centers treat their patients within the framework of studies of the ALL-BFM Study Group, an association of German, Austrian and Swiss pediatric oncology centers that has contributed significantly to therapy improvements in ALL in numerous large-scale randomized therapy studies since 1976. After most progress in the previous ALL-BFM trials was achieved through adjustments in risk grouping and individualization of treatment, promising new drugs are being used for the first time in the follow-up study AIEOP-BFM ALL 2017, which is now in planning. The so-called “backbone” of the treatment is represented by the aforementioned classical drugs of ALL treatment. In addition, new innovative drugs are randomized for potential benefit. The planned AIEOP-BFM-2017 study will define new cytogenetic high-risk groups (overview 1) that will have access to new innovative therapeutic approaches. One of these new subgroups is defined by the presence of an IKZF1 deletion in association with a CDKN2A, CDKN2B, PAX5, or PAR1 deletion in the absence of an ERG deletion and is referred to as IKZF1plus. In previous studies, approximately 10-15% of patients were identified as IKZF1plus and these had a significantly higher recurrence rate than the IKZF1plus-negative patients [8]. One of these newer drugs that will be used in a small group of high-risk groups with particularly unfavorable prognosis is blinatumomab, a bispecific T-cell antibody (BiTE) that simultaneously targets the T-cell CD3 receptor and the B-cell surface protein CD19 [9]. Blinatumomab is expected to combine two potential effects: Reduction of acute and long-term toxicities by saving conventional chemotherapy and more effective therapy for patients who have had an unsatisfactory response to high-risk therapy.
Another new drug with a novel mode of action in frontline ALL therapy is the proteasome inhibitor bortezomib. Because previous attempts at late intensification of therapy in high-risk patients have had little success, and because of already high toxicities of high-risk (HR) therapies, bortezomib will be randomized to HR patients in the early post-reinduction phase in the upcoming trial.
Central nervous system (CNS)
Prevention of CNS recurrence is nowadays mostly done by drugs, on the one hand with intrathecal methotrexate injections, on the other hand by administration of systemically acting cytostatic drugs infiltrating the brain (e.g. high-dose methotrexate). This has made it possible to limit previous CNS radiotherapy, which led to a dramatic reduction in CNS recurrences but was associated with not insignificant late effects, to very specific risk situations [10,11].
Stem cell transplantation
As the results of primary therapy as well as relapse protocols of leukemias have improved significantly over time, this has also led to a continuous adjustment of the indication for high-dose therapies with stem cell reinfusion. The current indication of stem cell transplantation (SZT) as part of primary therapy is reserved for certain prognostically unfavorable cytogenetic subgroups such as t(9;22), hypodiploidies with fewer than 44 chromosomes in the blasts, and IKZF1plus in combination with insufficient response to therapy (MRD) over time [12]. From the experience of the BFM group, it has been shown that the treatment success of relapses depends on the time of occurrence of the relapse, the attack pattern of the leukemia, and the leukemia subtype [13]. However, it has also been demonstrated that the response to therapy after renewed induction of therapy and thus the dynamics of the decline in minimal residual disease is of particular prognostic importance and accordingly further elements of therapy, such as the use of SCT, can be directed accordingly [14].
New therapies
With few exceptions (clofarabine, nelarabine, imatinib), there have been no new approvals for pediatric ALL in the last decade to 15 years. However, several interesting therapeutic approaches are currently under clinical investigation in phase I-III trials. In addition to the aforementioned blinatumomab, these include chimeric antigen receptor (CAR) T cells, which have already been used to successfully treat CD19-positive ALL relapses. Again, this is an immunotherapy that harnesses the potential of autologous cytotoxic T cells to recognize and destroy cells of the B-cell lineage. Other promising immunotherapies, some of them coupled with cytostatics, are currently in phase I/II trials. In addition to immunotherapies, targeted therapies after prior in vitro testing in xenograft or cell line models or specific inhibitors against cytogenetically detected fusion genes represent interesting and promising therapeutic options.
Take-Home Messages
- Acute lymphoblastic leukemia, the most common childhood cancer, is treated in a risk-adapted manner and is curable in the majority of cases.
- Determination of minimal residual disease after therapy induction is one of the most important prognostic factors along with biological markers such as leukemic subtype and cytogenetic and molecular genetic alterations of leukemic blasts.
- Current developments aim at a more effective and targeted treatment of previously resistant leukemia subtypes as well as a reduction of therapy toxicity.
- New innovative drugs such as immunotherapies and individualized therapeutic approaches are in clinical trials.
Literature:
- Jabbour E, et al: New insights into the pathophysiology and therapy of adult acute lymphoblastic leukemia. Cancer 2015; 121(15): 2517-2528.
- Pui CH, et al: Biology, risk stratification, and therapy of pediatric acute leukemias: an update. J Clin Oncol 2011; 29(5): 551-565.
- Kinlen L, et al: Infections and immune factors in cancer: the role of epidemiology. Oncogene 2004; 23: 60-75.
- Greaves M, et al: Infection, immune responses and the aetiology of childhood leukaemia. Nat Rev Cancer 2006; 6(3): 193-203.
- Campano D, et al: Minimal residual disease monitoring in childhood acute lymphoblastic leukemia. Curr Opin Hematol 2012; 19: 313-318.
- Conter V, et al: Molecular response to treatment redefines all prognostic factors in children and adolescents with B-cell precursor acute lymphoblastic leukemia: results in 3184 patients of the AIEOP-BFM ALL 2000 study. Blood 2010; 115(16): 3206-3214.
- Loh ML, et al: Tyrosine kinome sequencing of pediatric acute lymphoblastic leukemia: a report from the Children’s Oncology Group TARGET Project. Blood 2013; 121(3): 485-488.
- Hinze L, et al: Prognostic impact of IKZF1 deletions in association with vincristine-dexamethasone pulses during maintenance treatment of childhood acute lymphoblastic leukemia on trial ALL-BFM 95. Leukemia 2017; 31: 1840-1842.
- Brentjens RJ, et al: Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias. Blood 2011; 118(18): 4817-4828.
- Möricke A, et al: Risk-adjusted therapy of acute lymphoblastic leukemia can decrease treatment burden and improve survival: treatment results of 2169 unselected pediatric and adolescent patients enrolled in the trial ALL-BFM 95. Blood 2008; 111(9): 4477-4489.
- Kamps WA, et al: BFM-oriented treatment for children with acute lymphoblastic leukemia without cranial irradiation and treatment reduction for standard risk patients: results of DCLSG protocol ALL-8 (1991-1996). Leukemia 2002; 16(6): 1099-1111.
- Balduzzi A, et al: Chemotherapy versus allogeneic transplantation for very-high-risk childhood acute lymphoblastic leukaemia in first complete remission: comparison by genetic randomisation in an international prospective study. Lancet 2005; 366: 635-642.
- Tallen G, et al: Long-term outcome in children with relapsed acute lymphoblastic leukemia after time-point and site-of-relapse stratification and intensified short-course multidrug chemotherapy: results of trial ALL-REZ BFM 90. J Clin Oncol 2010; 28: 2339-2347.
- Eckert C, et al: Minimal residual disease after induction is the strongest predictor of prognosis in intermediate risk relapsed acute lymphoblastic leukaemia – Long-term results of trial ALL-REZ BFM P95/96. Eur J Cancer 2013 Apr; 49(6): 1346-1355.
InFo ONCOLOGY & HEMATOLOGY 2017; 5(5): 30-33.