New results from the FIREFISH and SUNFISH studies suggest that there is hope for children with spinal muscular atrophy.
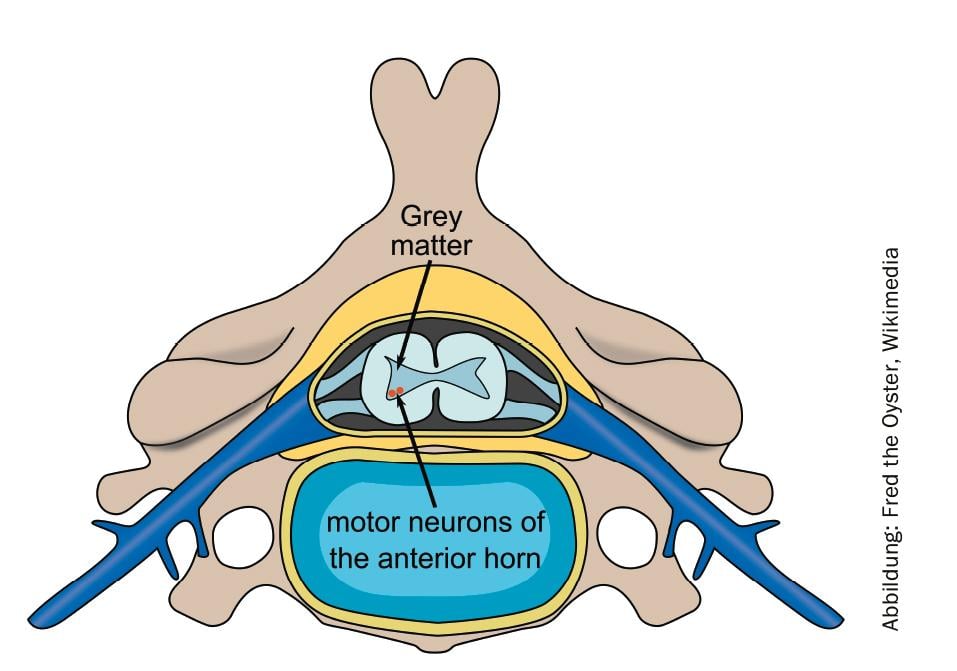
Spinal muscular atrophy (SMA) is a hereditary, severe and progressive neuromuscular disease that leads to muscle wasting and disease-related complications. Among rare diseases, SMA is the most common, affecting about one in 11,000 babies. The progressive loss of nerve cells in the spinal cord leads to motor impairments, the severity of which depends on the form of the SMA.
One promising therapeutic approach is risdiplam. The pyridazine derivative increases the functional survival of motor neuron life maintenance (SMN) genes by altering the splicing pattern. The compound is administered orally and is currently being investigated in the FIREFISH, SUNFISH, JEWELFISH and RAINBOWFISH studies. Roche presented new results from two studies at the AAN Annual Meeting in Philadelphia.
Another step towards oral therapy
FIREFISH is a two-part open-label pivotal study in children with type 1 SMA. Part 1 was a dose-escalation study in 21 children whose primary objective was to determine the safety profile of risdiplam. In addition, it should define the dose for part 2 (n=41). Part 1 showed that patients treated with risdiplam survived longer than untreated patients. In addition, they achieved motor milestones above what would be expected in a natural history of the disease. Thus, after 12 months, of 17 children who received the agent and were also randomized to Part 2, 7 children were able to sit without support for at least five seconds, 11 children were able to sit with/without support, and 9 were able to hold their heads upright. One child could stand. Side effects included, in decreasing frequency, fever (52%), upper respiratory tract infection (43%), diarrhea (29%), vomiting, cough, pneumonia, and constipation. No child required a tracheostomy or continuous ventilation, and none lost the ability to swallow. Three children died during the study, although these cases were not associated with risdiplam.
Overall, 59% of patients >scored 40 on the CHOP-INTEND scale, with a maximum of 57. The lead investigator, Giovanni Baranello, MD, of the Carlo Besta Neurological Research Institute Foundation in Milan (I), then emphasized that these positive results advocate a therapeutic approach that focuses on increasing SMN genes in the central nervous system as well as throughout the body. “We are very encouraged by these recent findings regarding risdiplam,” echoed Sandra Horning, MD, Roche’s chief medical officer and head of Global Product Development. “These bring us one step closer to being able to provide the first oral therapeutic option to the SMA community.” Part 2 of FIREFISH, designed to measure efficacy and including 41 patients, is currently underway.
Preparation of the efficacy study completed
SUNFISH, another two-part, double-blind, placebo-controlled, pivotal clinical trial, included 12-25-year-old patients with type 2 or type 3 SMA. The goal of Part 1 (n=51) was to determine the dose for Part 2. Part 2 primarily aims to evaluate motor skills at 12 months as measured by the Motor Function Measure 32 (MFM-32) score. While Part 2 (n=180) is currently being conducted, the results of Part 1 are available.
At baseline, the participants showed very different motor skills and limitations. Some no longer sit upright, while others still walk. In some, the scoliosis was severe; in others, it was not.
After twelve months of treatment with risdiplam, the median concentration of SMN protein in the blood was doubled. Forty-three patients were able to participate in all measurements, with 58% improving by 3 points in the MFM-32 scale at >.
Part 2 of both the FIREFISH and SUNFISH studies are now testing the efficacy of risdiplam. Results are expected in the fourth quarter of 2019 and the first quarter of 2020.
InFo NEUROLOGY & PSYCHIATRY 2019; 17(3): 40.