Chronic pruritus (> 6 weeks of duration) is one of the most frequent skin complaints and occurs in a broad variety of diseases including dermatological, systemic, neurological, psychosomatic/psychiatric and gynaecological ones (1). According to the criteria of Hanifin et al., pruritus is an essential feature of atopic dermatitis (AD) (2). While in AD all patients suffer from pruritus, 80% of psoriasis patients have this complaint (3). Pruritus of high intensity is common in AD, (4), whereas in ichthyosis and psoriasis moderate pruritus is frequently observed(5, 6). A large questionnaire survey revealed that AD patients experience itch more frequently at night, in the evening and during the winter (4). More than half the surveyed participants reported pain (59%) and heat sensation (53%) associated with itch, which are significantly correlated with itch intensity (4). Pruritus as well as eczema in AD has a high impact on the quality of life (7,8). The Skindex comprising 16 questions has been recently developed as a specific instrument for measuring health-related quality of life (HRQoL) in dermatology patients (8). A recent study showed that improvement of AD parallels the reduction of Skindex value. In particular, the emotions score of Skindex significantly correlates with reduction of pruritus intensity (8).
Pathophysiology of atopic dermatitis pruritus
The pathophysiology of pruritus in AD is still not fully understood (9). During the past years, new data have emerged which revealed novel aspects of the molecular mechanisms of itch in AD, such as hyperplasia of cutaneous nerves, AD-specific interleukin (IL-31) and histamine H4 receptor expression on inflammatory cells (9). Several of these mechanisms are involved in the pathophysiological changes in the epidermis and contribute to barrier disruption. For example, activation of the thermoreceptor transient receptor potential vanilloid 1 (TRPV1) has an effect on the epidermal barrier state. In an animal study it was demonstrated that the skin surface temperature has an influence on epidermal permeability barrier (10). Temperatures of 42 ºC, for instance, led to a delayed barrier recovery. Capsaicin activated the TRPV1 and thereby delayed barrier recovery, whereas capsazepin, an antagonist of TRPV1, blocked this delay (10). This suggested that TRPV1 is involved in epidermal barrier homeostasis. TRPV1 is expressed on keratinocytes and sensory nerves (1). Activation of TRPV1 has, therefore, a double effect in AD namely, induction of itch and delayed epidermal barrier recovery (Table 1).
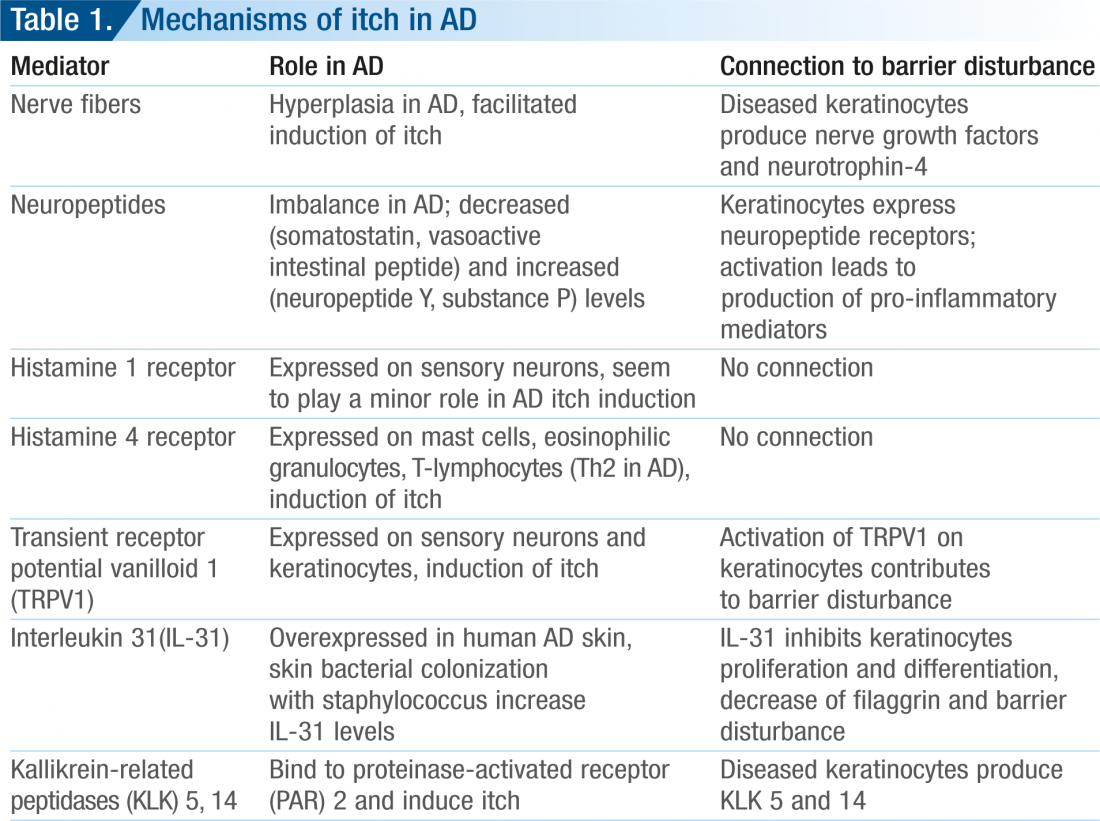
The long-lasting and intense pruritus usually occurring in AD may be explained by alterations in the number of cutaneous nerve fibres in AD skin lesions (11). In lesional AD skin, increased numbers of neuropeptidecontaining nerve fibres such as calcitonin gene-related peptide (CGRP) and substance P (SP) have been demonstrated (12, 13). Hypertrophy of
nerve fibres in AD is stimulated by an increased release of nerve growth factors secreted by basal keratinocytes and inflammatory cells. For example, neurotrophin-4 (NT-4) and nerve growth factor (NGF) are keratinocyte-derived factors which are highly expressed under inflammatory conditions and exert growth-promoting effects on nerve cells. In lesional skin of patients with AD, NT-4 and NGF expression was found to be significantly increased (14, 15).
The newly discovered interleukin, interleukin (IL)-31 might have a major role in the pathophysiology of AD pruritus (16). IL-31 was significantly overexpressed in human AD skin compared with nonpruritic psoriatic skin inflammation (17). IL-31 receptors are expressed on sensory neurons (detected on dorsal root ganglia). Accordingly, binding of IL-31 on sensory neurons leads to induction of itch in AD (17). A recent study demonstrated in a 3-dimensional organotypic skin model that IL-31 interferes also with keratinocytes and their differentiation (18). IL-31 inhibited proliferation of keratinocytes and repression of terminal differentiation markers, including filaggrin (FLG) (18). Loss of function of FLG is of great significance for barrier impairment in AD and ichthyosis vulgaris (IV) (19). Accordingly, high IL-31 levels in atopic skin may promote not only pruritus but also a disturbed skin barrier (18). Recently, histamine 4 receptors (H4) were found on inflammatory cells including Th2 lymphocytes of AD patients (20). Stimulation of H4 receptor leads to up-regulation of IL-31 in Th2 lymphocytes. This newly found mechanism may explain the quick increase of pruritus intensity during a flare-up in AD patients. Interestingly, a mouse model suggests that a combination of H4 and H1 receptor antagonism might be a new strategy to treat pruritus related to allergic diseases like AD (21). In their experiments, the authors showed that H4 receptor antagonism failed to reduce the allergic inflammatory response but strongly inhibited allergen-induced itch (21). In sum, the complex pathophysiology of itch in AD involves several mediators and receptors. They are connected by a functional cascade and their action leads frequently to induction of pruritus, and disturbed epidermal barrier, independent of the genetically determined filaggrin disturbance. An important step in the therapy of AD is, therefore, the restoration of the epidermal barrier.
Management of AD pruritus: limits of steroids and antihistamines
Since several studies have demonstrated that different, non-histamine receptor 1-mediated mechanisms are involved in AD, it is not surprising that conventional therapeutic modalities like antihistamines often fail to ameliorate pruritus in AD (22). Placebo-controlled studies concerning the antipruritic effect of oral antihistamines have shown conflicting results in AD. In some studies, antihistamines demonstrated no superior effect compared to placebo while in others, they showed a significant antipruritic effect (23, 24). An evidence-based review of the efficacy of antihistamines in relieving pruritus in AD concluded that little objective evidence exists for the antipruritic efficacy of H1-antihistamines (22).
Topical steroids are frequently used in the treatment of AD. They are able to induce a fast relief of pruritus in AD. However, there is no strong evidence for their direct antipruritic effects (25). Their long-term use, however, can result in skin atrophy and fragility, which can then itself trigger the pruritic disposition. To date, no substances targeting IL-31 or H4 are available. The first and very important step in the reduction and prevention of itch in AD is to counterbalance the barrier disturbance.
Preventive role of emollients
Fillagrin is an important differentiation factor in the stratum granulosum. Natural moisturizing factor (NMF) is a product of filaggrin degradation in the stratum corneum (26). NMFs are reduced in AD skin with a genetically determined filaggrin dysfunction and promote barrier disturbance. However, in all patients with AD, barrier disturbance with clinically dry and scaly skin is a common observation. A topical emollient therapy to restore skin barrier function, to reduce trans-epidermal water loss, to improve uncomfortable cutaneous dry skin and itch, moisturization of the skin is very important in AD. To achieve this, a daily emollient use is necessary. Modern emollients avoid fragrances and preserving agents which may induce allergies. For relief of itch, some emollients contain urea and polidocanol. This is very useful in adults but its application is limited in children (9). A cream containing 15% glycerol, 8% white-soft parafin and 2% and liquid paraffin (Dexeryl®, Laboratoires Pierre Fabre, Castres, France) is a new product that can help counterbalance the barrier disturbance. Glycerol is a water-soluble substance which is absorbed into the stratum corneum. In the vital layers of the epidermis, it increases the degree of hydration and exhibits a regenerative barrier repair effect (6, 27, 28). Due to its ingredients, this emollient can be applied in both adults and children. One recent study including 265 children with ichthyosis demonstrated safety and good tolerance of the glycerol and paraffin-containing emollient (6). In children, after a 4-week use of the emollient, skin scaling and pruritus intensity improved significantly (6). The antipruritic effects of the glycerol and paraffincontaining cream were also demonstrated in another study (29). Patients with pruritus due to chronic kidney disease applied the emollient twice daily for 7 days; 73% of patients reported significant reduction of itch compared to a vehicle control (p < 0.0001). These data are very encouraging and suggest that use of this emollient may be beneficial in patients with dry skin and pruritus not only related to AD but also other underlying diseases. Its safety profile for use in children is a special advantage, since administration of systemic antipruritic acting substances such as gabapentin known to have their adverse side effects in this patient group can be dispensed with.
References
- Ständer S, Raap U, Weisshaar E, Schmelz M, Mettang T, Handwerker H, Luger TA. Pathogenese des Pruritus. J Dtsch Dermatol Ges. 2011; 9: 456-463.
- Hanifin JM, Rajka G. Diagnostic features of atopic dermatitis. Acta Derm Venereol (Stockh) 1980;92(suppl):44-47.
- Ständer S, Grundmann SA, Luger TA. Chronic Pruritus. Giornale Italiano di Dermatologia e Venereologia 2012, n press.
- Dawn A, Papoiu AD, Chan YH, Rapp SR, Rassette N, Yosipovitch G.Itch characteristics in atopic dermatitis: results of a web-based questionnaire. Br J Dermatol. 2009 Mar; 160: 642-4. Amatya B, Wennersten G, Nordlind K. Patients perspective of pruritus in chronic plaqueb psoriasis: a questionnaire-based study. J Eur Acad Dermatol Venereol 2008;22:822-826.
- Blanchet-Bardon C, Tadini G, Machado Matos M, Delarue A. Association of glycerol and paraffin in the treatment of ichthyosis in children: an international, multicentric, randomized, controlled, double-blind study. J Eur Acad Dermatol Venereol. 2011 in press, doi:
- 1111/j.1468-3083.2011.04304.x. [Epub ahead of print].
- Maksimović N, Janković S, Marinković J, Sekulović LK, Zivković Z, Spirić VT. Health-related quality of life in patients with atopic dermatitis. J Dermatol. 2011, in press doi: 10.1111/j.1346-8138.2011.01295.x. [Epub ahead of print].
- Kawakami T, Kimura S, Haga T, Doi R, Kyoya M, Nakagawa K, Soma Y. Health-related quality of life assessed by the effect of bepotastine besilate in patients with pruritus: Importance of emotions score in atopic dermatitis. J Dermatol. 2011 in press. doi: 10.1111/j.1346-8138.2011.01418.x. [Epub ahead of print].
- Ständer S, Luger T. Itch in atopic dermatitis – Pathophysiology and treatment. Acta Dermatovenerol Croat 2010; 18: 289-96.
- Denda M, Sokabe T, Fukumi-Tominaga T, Tominaga M. Effects of skin surface temperature on epidermal permeability barrier homeostasis. J Invest Dermatol 2007:127:654-659.
- Tobin D, Nabarro G, de la Faille HB, van Vloten WA, van der Putte SCJ, Schuurman HJ. Increased number of immunoreactive nerve fibers in atopic dermatitis. J Allergy Clin Immunol 1992;90:613-622.
- Urashima R, Mihara M. Cutaneous nerves in atopic dermatitis. A histological, immunohistochemical and electron microscopic study. Virchows Arch 1998;432:363-370.
- 13. Pincelli C, Fantini F, Massimi P, Girolomoni G, Seidenari S, Giannetti A. Neuropeptides in skin from patients with atopic dermatitis: an immunohistochemical study. Br J Dermatol 1990;122:745-750.
- Grewe M, Vogelsang K, Ruzicka T, Stege H, Krutmann J. Neurotrophin-4 production by human epidermal keratinocytes: increased expression in atopic dermatitis. J Invest Dermatol2000;114:1108-12.
- Tominaga M, Tengara S, Kamo A, Ogawa H, Takamori K. Psoralen-ultraviolet A therapy alters epidermal Sema3A and NGF levels and modulates epidermal innervation in atopic dermatitis. J Dermatol Sci 2009;55:40-6.
- Takaoka A, Arai I, Sugimoto M, Yamaguchi A, Tanaka M, Nakaike S. Expression of IL-31 gene transcripts in NC/Nga mice with atopic dermatitis. Eur J Pharmacol 2005;516:180-81.
- Sonkoly E, Muller A, Lauerma AI, Pivarcsi A, Soto H, Kemeny L, et al. IL-31: a new link between T cells and pruritus in atopic skin inflammation. J Allergy Clin Immunol 2006;117:411-17.
- Cornelissen C, Marquardt Y, Czaja K, Wenzel J, Frank J, Lüscher-Firzlaff J, Lüscher B, Baron JM. IL-31 regulates differentiation and filaggrin expression in human organotypic skin models. J Allergy Clin Immunol. 2011, in press [Epub ahead of print].
- Winge MC, Hoppe T, Berne B, Vahlquist A, Nordenskjöld M, Bradley M, Törmä H. Filaggrin genotype determines functional and molecular alterations in skin of patients with atopic dermatitis and ichthyosis vulgaris. PLoS One. 2011;6(12):e28254.
- Gutzmer R, Mommert S, Gschwandtner M, Zwingmann K, Stark H, Werfel T. The histamine H4 receptor is functionally expressed on T(H)2 cells. J Allergy Clin Immunol. 2009;123:619-25.
- Roßbach K, Wendorff S, Sander K, Stark H, Gutzmer R, Werfel T, et al. Histamine H(4) receptor antagonism reduces hapten-induced scratching behaviour but not inflammation. Exp Dermatol 2009;18:57-63.
- Klein PA, Clark RAF. An evidence-based review of the efficacy of antihistamines in reliefing pruritus in atopic dermatitis. Arch Dermatol 1999;135: 1522-1525.
- Wahlgren CF, Hägermark Ö, Bergström R. The antipruritic effect of a sedative and a nonsedative antihistamine in atopic dermatitis. Br J Derm 1990;122:545-551.
- Hannuksela M, Kalimo K, Lammintausta K, Turjanmaa K, Vajionen E, Coulie PJ. Dose ranging study: cetirizine in the treatment of atopic dermatitis in adults. Ann Allergy 1993;70:127-33.
- Zhai H, Frisch S, Pelosi A, Neibart S, Maibach HI. Antipruritic and thermal sensation effects of hydrocortisone creams in human skin. Skin Pharmacol Appl Skin Physiol. 2000;13:352-7.
- Novak N, Leung DY. Advances in atopic dermatitis. Curr Opin Immunol. 2011 Dec;23(6):778-83.
- Froebe C, Simion FA, Ohlmeyer H et al. Prevention of stratum corneum lipid phase transitions in vitro by glycerol – an alternative mechanism for skin moisturization. J Soc Cosmet Chem 1990; 41: 51–65.
- Fluhr JW, Gloor M, Lehmann L, Lazzerini S, Distante F, Berardesca E. Glycerol accelerates recovery of barrier function in vivo. Acta Derm Venereol 1999; 79: 418–421.
- Szepietowski JC, Reich A, Schwartz RA. Uraemic xerosis. Nephrol Dial Transplant. 2004; 19:2709-2712.