Research into new biologics is helping to provide treatment alternatives for moderate to severe plaque psoriasis in the event of a lack of response or loss of efficacy [1]. That rapid improvement in skin symptoms and freedom from lesions are among the most important criteria for patients is shown by data published in 2019 by the Swiss Dermatology Network of Targeted Therapies (SDTT), said Julia-Tatjana Maul, MD, University Hospital Zurich [2,3].
Back to “Atopic dermatitis and psoriasis news”.
The interleukin-23 inhibitor risankizumab (SKYRIZITM) has been approved in Switzerland since April 2019 for moderate-to-severe plaque psoriasis after unsuccessful therapy with conventional systemic treatment [4]. In addition, as of August 1, 2019, the humanized monoclonal antibody is eligible for health insurance coverage [5]. Risankizumab is injected subcutaneously (150 mg; 2× 75 mg); at four-week intervals after the first administration and at twelve-week intervals thereafter [4]. The high and long-lasting efficacy with rapid response as well as good tolerability have been clinically confirmed [6–8].
Long-term data: Positive conclusion
The high efficacy, practicability and tolerability contribute to an improvement in quality of life, which is also an important therapeutic goal, according to Prof. Peter Häusermann, MD, University Hospital Basel. Thanks to the rapid response rate – significant symptom relief within 4 to 8 weeks – the phase of psoriasis-related impairment of everyday life can be reduced. The expert highlighted that the efficacy of risankizumab does not diminish even with prolonged use [9]. This is also supported by data from various phase III studies [6–8].
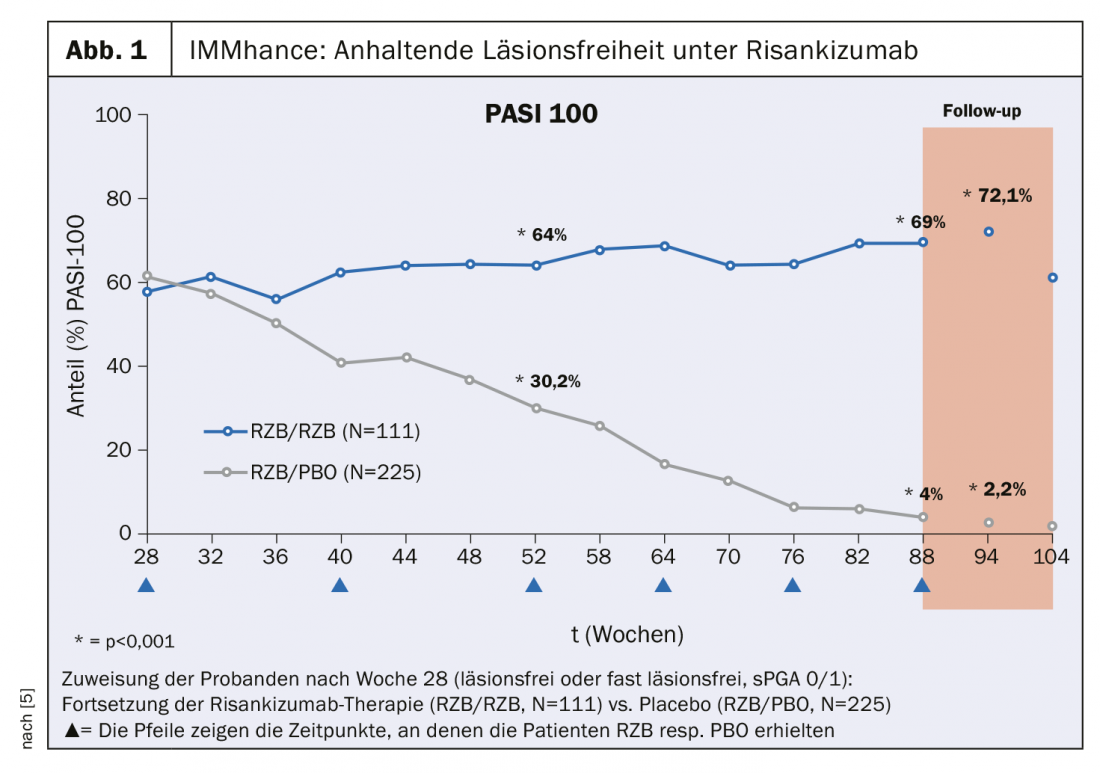
The IMMhance study investigated the long-term effects of interrupting risankizumab therapy in patients with moderate to severe plaque psoriasis. Those who achieved a lesion-free or near-lesion-free status at week 28 after treatment initiation (Static Physician Global Assessment, sPGA 0/1) were randomly assigned to the conditions continuation of risankizumab therapy (n=111) vs placebo (n=225). In the group of study participants still treated with risankizumab, more than 70% achieved complete skin healing according to Psoriasis Area Severity Index (PASI) 100 at week 94 (Fig. 1). In contrast, in the group switched to placebo, the PASI 100 response worsened from 61.3% to 2.2% [6]. In the UltIMMa-1 and UltIMMa-2 trials, according to an integrated analysis of both studies, 75.1% and 81.3% of risankizumab-treated patients (n=598) achieved a PASI 90 after 16 and 52 weeks of treatment, respectively, which is significantly more than in the ustekinumab group, in which less than 50% of 199 subjects achieved a PASI 90 at both time points [8].
Health economics controversy
Risankizumab is indicated for the treatment of moderate to severe plaque psoriasis in adults who have had an inadequate response to other systemic therapies [4]. Specifically, at least one conventional systemic therapy (ciclosporin, methotrexate, acitretin, or UVB and PUVA) must have failed after 16 weeks of treatment [5]. There is no consensus on how many systemic therapy trials with nonbiologics should be tried beforehand. In general, however, a personalized therapy strategy is propagated. Whether to stop therapy when a PASI of 100 is reached and what is considered a criterion for switching to another biologic are evaluated differently. Prof. Häusermann has the vision that in about five years the innovative substance class of IL-23 inhibitors will be used routinely. By then, it may also be possible to narrow the price gap with conventional systemic therapeutics, which is currently a health economic dilemma for these highly effective drugs [9].
Back to “Atopic dermatitis and psoriasis news”.
Literature:
- Birchler T: Risankizumab as a treatment option for psoriasis. Slide presentation. Thomas Birchler, AbbVie AG, Medical Advisor, Media Roundtable, Iaculis GmbH & AbbVie AG, Oct. 29, 2019, Zurich.
- Maul JT: Risankizumab as a treatment option for psoriasis. Slide presentation. Julia-Tatjana Maul, MD, Media Roundtable, Iaculis GmbH & AbbVie AG, Oct. 29, 2019, Zurich.
- Maul JT et al. Gender and age significantly determine patient needs and treatment goals in psoriasis – a lesson for practice. J Eur Acad Dermatol Venereol, 2019. 33(4): p. 700-708.
- Current SKYRIZITM technical information at www.swissmedicinfo.ch.
- Specialty List 2019 of the Federal Office of Public Health FOPH. www.spezialitaetenliste.ch, as of August 2019.
- Blauvelt A, Leonardi, C., Gooderham, M., Papp, K. Efficacy and Safety of Continuous Q12W Risankizumab Versus Treatment Withdrawal: 2-Year Double-Blinded Results from the Phase 3 IMMhance Trial. presented at the 24th World Congress of Dermatology. Milan, Italy, 10-15 June 2019.
- Gordon KB et al. Efficacy and safety of risankizumab in moderate-to-severe plaque psoriasis (UltIMMa-1 and UltIMMa-2): results from two double-blind, randomised, placebo-controlled and ustekinumab-controlled phase 3 trials. Lancet, 2018. 392(10148): p. 650-661.
- Lebwohl M et al. Efficacy and Safety of Risankizumab in Moderate-to-Severe Plaque Psoriasis: An Integrated Analysis of UltIMMa-1 and UltIMMa-2. P8108, presented at the American Academy of Dermatology Annual Meeting, Washington DC, March 1-5, 2019.
- Häusermann P: Risankizumab as a treatment option for psoriasis. Slide presentation. Prof. Peter Häusermann, MD, Media Roundtable, Iaculis GmbH & AbbVie AG, Oct. 29, 2019, Zurich.
Adapted from: DERMATOLOGIE PRAXIS 2019; 29(6): 26











