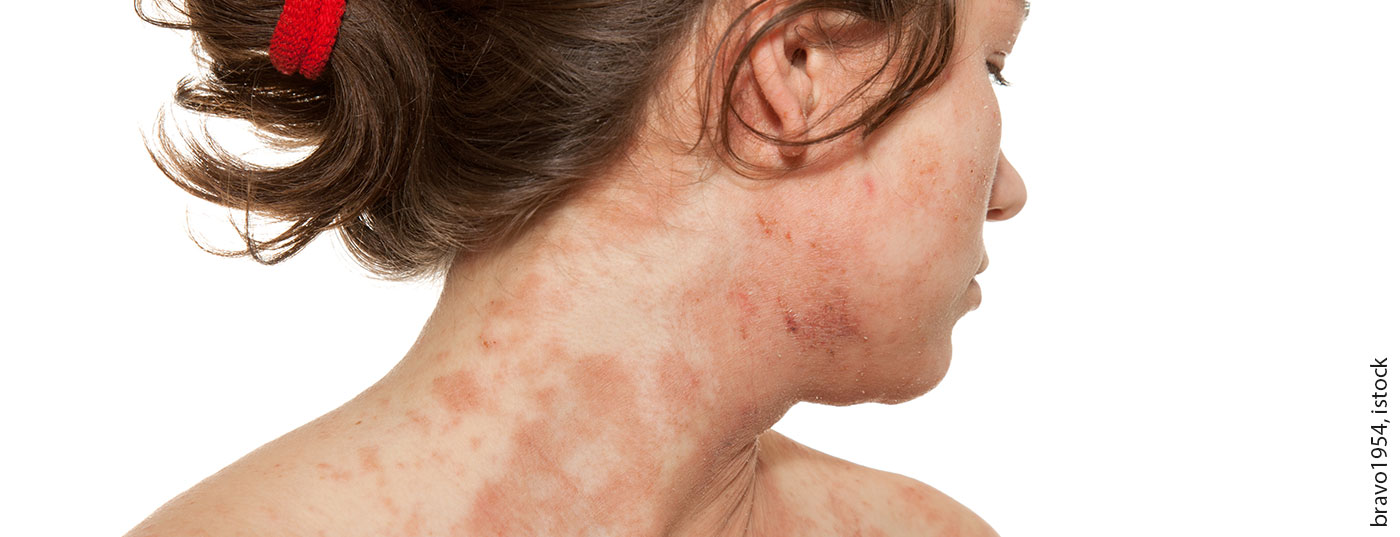
Th2-mediated inflammatory processes play a central pathogenetic role in atopic dermatitis. This is the therapeutic target of the IL4/IL13 inhibitor dupilumab, which was recently approved in Switzerland also for adolescents aged 12 years and older. The biologic specifically intervenes in the disease process and is therefore associated with fewer side effect risks compared to conventional system therapeutics.
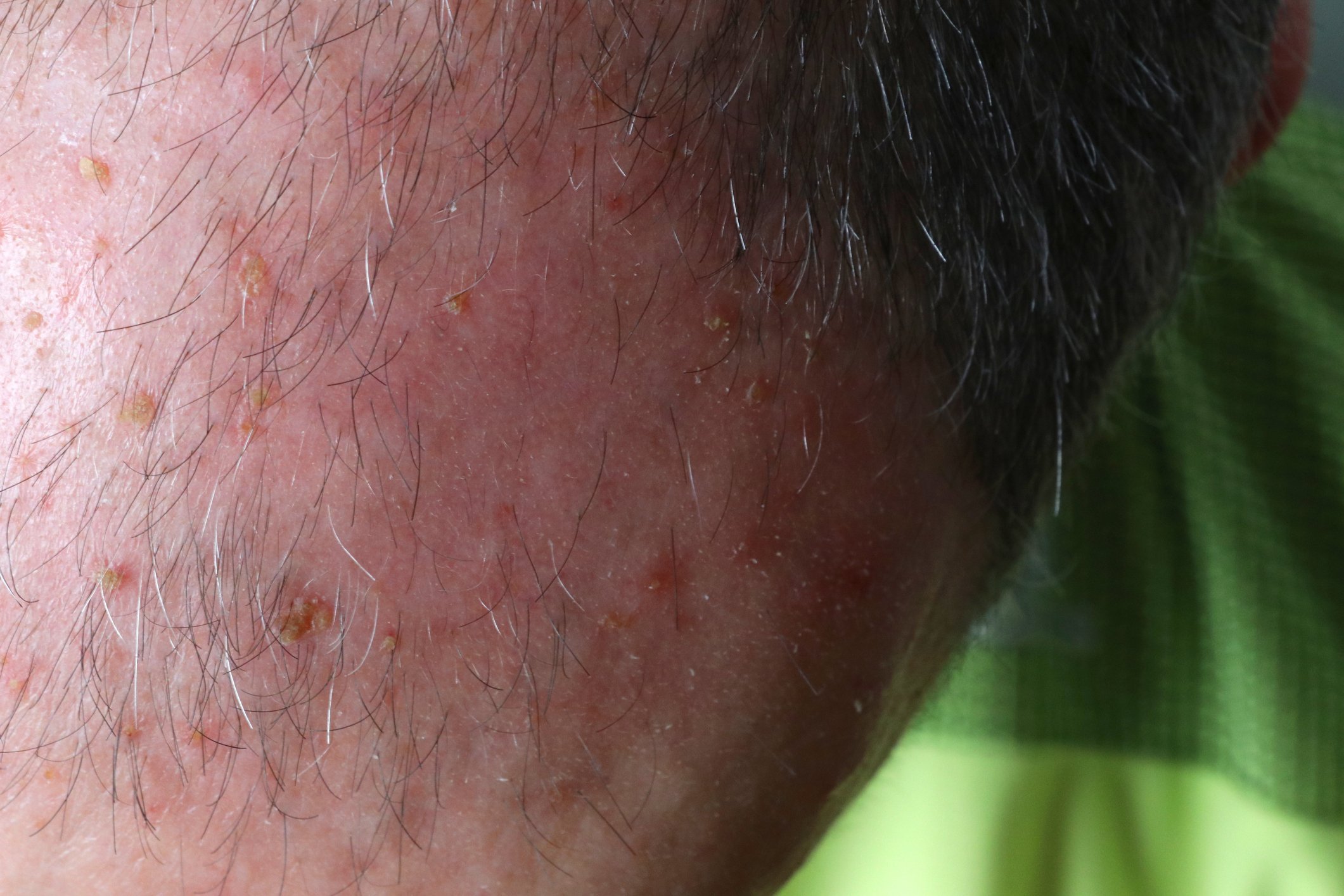
“Atopic dermatitis is one of the most common chronic diseases,” says Prof. Dagmar Simon, MD, Head of Dermatology, Inselspital Bern [1]. About 12-15% of children between 5 and 7 years and up to 8% of adults are affected, he said. Patients suffer from a significant “burden of disease”, especially when the disease is severe. Itching is very unpleasant, can lead to sleep disturbances and, especially in children and adolescents, can also contribute to concentration and learning difficulties. With visible skin changes, patients also feel very stigmatized. As we know from various empirical studies, mental disorders occur more frequently. According to a meta-analysis, the risk for depression and anxiety disorders is about twice as high as in healthy individuals (OR 2.19) [2]. It is known that this applies not only to adults but also to children, Prof. Simon said. Therapy that relieves itching and eczematous lesions also has a beneficial effect on the quality of life of those affected.
Dupilumab specifically intervenes in the inflammatory process
The pathophysiological interaction structure in atopic dermatitis is extremely complex. Th2-mediated inflammation and an epidermal barrier defect are the most important pathogenetic factors, explains Prof. Simon (box). The mechanism of action of the human monoclonal IgG4 antibody dupilumab is based on a reduction of the Th2-mediated inflammatory cascade. Inhibition of IL4 and IL13 signaling pathways downregulates Th2-mediated inflammatory processes [8,9]. Dupilumab (Dupixent®) provides rapid and sustained symptom relief and has been shown to improve quality of life. Patients benefit greatly from this treatment, as reflected by good adherence [23–25]. In November 2020, Dupixent® was also approved by Swissmedic for adolescents aged 12 years and older with moderate to severe atopic dermatitis (overview 1) .

The biologic’s target molecules, the Th2-associated cytokines IL4 and IL13, are instrumental in inflammatory processes by stimulating IgE antibody formation, which can fuel itch-scratch cycles, perpetuate inflammation, and further degrade barrier function [10].
At the molecular level, dupilumab leads to a reduction in the signature of over 800 genes involved in atopic dermatitis, including those for Th2 chemokines, T cell proliferation and dendritic cells [11]. Research into the various cellular targets of dupilumab is ongoing [12].
Excellent treatment results in adults and adolescents
Systemic treatment is prescribed in a step-adapted manner, in parallel with basic therapy [13]. In a new formulation of the ETFAD/EADV “Eczema Task Force” stepwise regimen for the treatment of atopic dermatitis published in 2020, biologics have now been included as a systemic therapy option, according to Prof. Simon [14]. In addition, recommendations for switching therapy from conventional immunosuppressants to dupilumab have been published [26]. From a pharmacological point of view, there are no objections to immediate follow-up therapy in line with the indication.
The results to date on dupilumab have been extremely positive. In the large clinical trials, the IL4/IL13 inhibitor showed excellent efficacy with low side effect rates. In the phase III pivotal SOLO1 and SOLO2 studies, 671 patients with moderate to severe atopic dermatitis received dupilumab 300 mg s.c. or placebo at weekly or biweekly intervals. After four months, an average of 37% of patients achieved significant clinical improvement to freedom from symptoms compared to 9% in the placebo group. A 75% improvement in EASI score was achieved in 52% of dupilumab-treated study participants after 16 weeks, compared with only up to 15% of placebo-treated participants [15–17]. Empirical studies in the “real world” setting were able to confirm efficacy and good tolerability of dupilumab [18–20].
Excellent treatment results were also achieved in adolescents: In a randomized-controlled double-blind study in 251 patients aged 12 to 17 years with moderate to severe atopic dermatitis, the IL4/IL13 inhibitor was shown to be very effective and safe [21]. EASI75 was achieved by 42% of adolescents treated every two weeks with dupilumab 200 mg or 300 mg, and 24% achieved an IGA of 0 or 1, compared with 8% and 2%, respectively, in the placebo group. An improvement in quality of life of at least 6 points on the Children’s Dermatology Life Quality Index (CDLQI) was seen in 61% of patients with dupilumab, compared with 20% in the placebo group [22].
For adolescents, weight-adapted dosing regimen.
In adults, dupilumab is administered as a subcutaneous injection (2× 300 mg) at an initial dose of 600 mg, followed by 300 mg at two-week intervals. In adolescents between 12 and 17 years of age, the administration interval is also two weeks, and the dosing regimen is weight-adapted (overview 2) . Biologics are not taken in tablet form, but are injected subcutaneously. Dupilumab (Dupixent®) is available as a pre-filled syringe in the prescribed dosage and can be self-administered by patients after medical instruction [8].

The risk of side effects with dupilumab is lower than with conventional systemic therapeutics such as ciclosporin, methotrexate, azathioprine, and mycophenolate mofetil due to a very targeted intervention in the disease process. A specific side effect of dupilumab is the occurrence of blepharitis and conjunctivitis. In most cases, these side effects are mild to moderate and can be improved with topical therapies or lengthening of injection intervals. A position paper on the management of conjunctivitis will be published soon, Prof. Simon said.
Congress: SGDV Dermatoallergology 2021
Literature:
- Simon D: Atopic dermatitis, Prof. Dr. med. Dagmar Simon, SGDV Dermatoallergologie, 18.03.2021
- Ronnstad ATM, et al: Association of atopic dermatitis with depression, anxiety, and suicidal ideation in children and adults: A systematic review and meta-analysis. JAAD 2018; 79: 448-456.
- Traidl S, Werfel T: Atopic dermatitis and internal medicine comorbidities. The Internist 2019; 60: 792-798.
- Jungersted J, et al: Stratum corneum lipids, skin barrier function and filaggrin mutations in patients with atopic eczema. Allergy 2010; 65: 911-918.
- Volz T, et al: On the role of the innate immune system in atopic dermatitis. Dermatologist 2015; 66: 90-95.
- Biedermann T, et al: Regulation of T Cell Immunity in Atopic Dermatitis by Micro-bes: The Yin and Yang of Cutaneous Inflammation. Front Immunol 2015; 6: 353.
- Chylla R, Schnopp C, Volz T: Basic therapy in atopic dermatitis – new and proven. JDDG 2018; 16 (8): 976-980.
- Brief technical information: Dupixent®, www.swissmedicinfo.ch (last accessed 19.03.2021)
- Del Rosso JQ: Monoclonal Antibody Therapies for atopic dermatitis: where are we now in the spectrum of disease management? J Clin Aesthet Dermatol 2019; 12(2): 39-41.
- AWMF: Guideline Neurodermatitis [atopic eczema; atopic dermatitis] Development stage: S2k. AWMF Register Number: 013-027 Long version, www.awmf.org (last accessed 03/19/2021).
- Hamilton JD, et al: Dupilumab improves the molecular signature in skin of patients with moderate-to-severe atopic dermatitis. J Allergy Clin Immunol 2014; 134(6): 1293-1300.
- Harb H, Chatila TA: Mechanisms of Dupilumab. Clinical & Experimental Allergy 2020; 50(1): 5-14.
- European Medicines Agency (EMA): Dupixent. Product information, summary of product characteristics, www.ema.europa.eu
- Wollenberg A, et al: ETFAD/EADV Eczema task force 2020 position paper on diagnosis and treatment of atopic dermatitis in adults and children. JEADV 2020; 34: 2717-2744.
- Blauvelt A, et al: Long-term management of moderate-to-severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): A 1-year, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017; 389(10086): 2287-2303.
- de Bruin-Weller M, et al: Dupilumab with concomitant topical corticosteroid treatment in adults with atopic dermatitis with an inade-quate response or intolerance to ciclosporin A or when this treatment is medically inadvisable: a placebo-controlled, randomized phase III clinical trial (LIBERTY AD CAFE). Br J Dermatol 2018; 178(5): 1083-1101.
- Simpson EL: Dupilumab improves general health-related quality-of-life in patients with moderate-to-severe atopic dermatitis: pooled results from two randomized, controlled phase 3 clinical trials. Dermatol Ther 2017; 7(2): 243-248.
- Uchida H, et al: Real-world effectiveness and safety of dupilumab for the treatment of atopic dermatitis in Japanese patients: A single-centre retrospective study. Br J Dermatol 2019, https://doi.org/10.1111/bjd.18163
- Olesen CM, et al: Treatment of atopic dermatitis with dupilumab: experience from a tertiary referral centre. J Eur Acad Dermatol Venereol 2019, https://doi.org/10.1111/jdv.15609
- Quint T, et al: Dupilumab for the treatment of atopic dermatitis in an Austrian cohort-real-life data shows rosacea-like folliculitis. J Clin Med 2020, https://doi.org/10.3390/jcm9041241
- Simpson EL: Efficacy and Safety of Dupilumab in Adolescents With Uncontrolled Moderate to Severe Atopic Dermatitis. A Phase 3 Randomized Clinical Trial. JAMA Dermatol 2020; 156; 44-56.
- Paller AS, et al: Clinically Meaningful Responses to Dupilumab in Adolescents with Uncontrolled Mode-rate-to-Severe Atopic Dermatitis: Post-hoc Analyses from a Randomized Clinical Trial. Am J Clin Dermatol. 2020; 21(1): 119-131.
- Simpson EL, et al: Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med 2016; 375: 2335-2348.
- Thaci: Efficacy and safety of dupilumab monotherapy in adults with moderate-to-severe atopic dermatitis: a pooled analysis of two phase 3 randomized trials (LIBERTY AD SOLO 1 and LI-BERTY AD SOLO 2). J Dermatol Sci 2019: 266-275.
- Worm M, et al: Modern therapy of atopic dermatitis: biologics and small-molecule drugs. JDDG 2020; 18: 10: 1085-1093.
- Wohlrab J, Mrowietz U, Weidinger S, et al: Recommended course of action for switching therapy from immunosuppressants to dupilumab in patients with atopic dermatitis. Dermatologist (2020). https://doi.org/10.1007/s00105-020-04720-1
DERMATOLOGIE PRAXIS 2021; 31(2): 26-27 (published 4/13/21, ahead of print).