Restless legs syndrome is one of the most common neurological disorders, with a prevalence of approximately 7% – an update.
Restless legs syndrome (RLS) is one of the most common neurological diseases with a prevalence of about 7%. The first convincing description of the complaints is attributed to the English anatomist Thomas Willis (1672). A more detailed typification of the RLS symptom complex was made in the 19th century by Theodor Wittmaack, among others, who introduced the term “Anxietas tibiarum” due to the urge to move. In the first half of the 20th century, familial clustering and exacerbation by pregnancy were recognized, but it was not until 1944-1945 that Karl-Axel Ekbom summarized all the clinical features and coined the name RLS that is commonly used today [1]. RLS is often also referred to as “Willis-Ekbom disease” (WED) (Fig. 1). Since RLS can affect not only the legs but also the arms, the name WED or RLS/WED has recently been proposed again.

Complaints and diagnostics
Affected patients often have difficulty describing their RLS symptoms. Sensory symptoms include dysesthesias, burning, pulling, tingling, electrification, or itching. Half of the patients experience pain. The decisive feature, however, is the urge to move, which does not necessarily have to be accompanied by sensations of discomfort. RLS is a clinical diagnosis; in addition to the urge to move, essential RLS features include occurrence at rest, worsening in the evening and at night, and improvement with movement (see box “Diagnostic RLS criteria”).

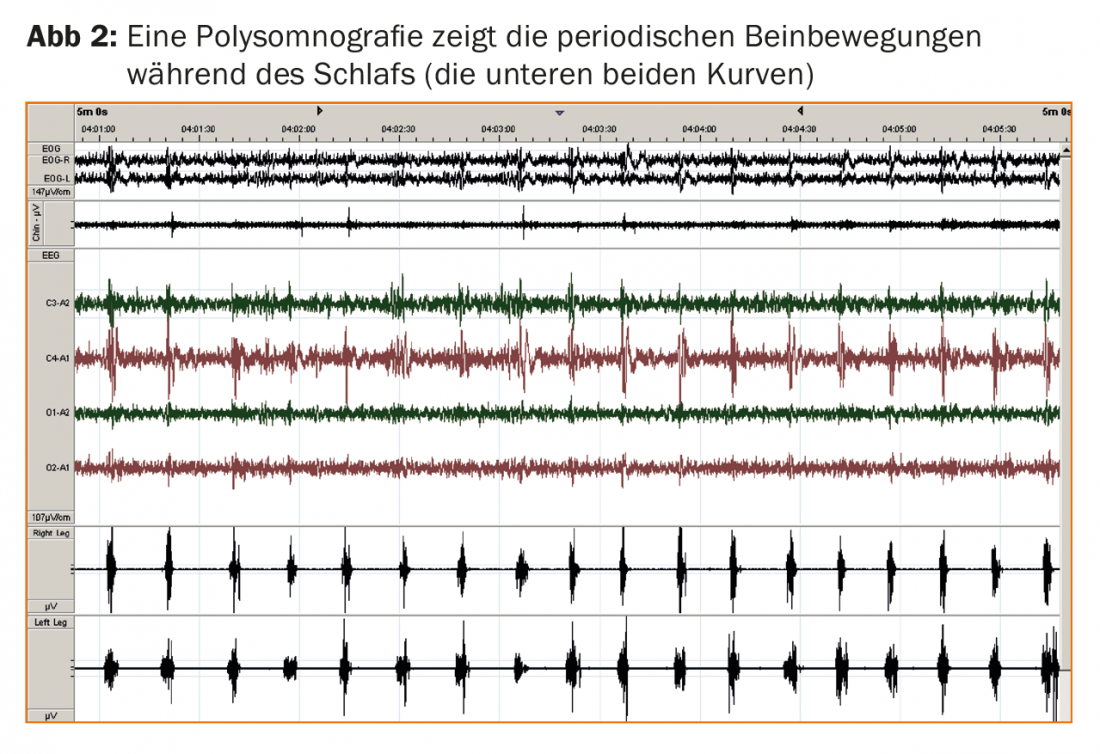
Polysomnography is an optional but useful diagnostic adjunct. Polysomnography allows detection of periodic leg movements during sleep (PLMS; Fig. 2) in almost all RLS patients and captures the effects on the quality of nighttime sleep, typically in the form of prolonged latency to fall asleep and sleep fragmentation due to PLMS-induced cortical arousal responses. The immediate response to low-dose levodopa and the positive family history in more than 50% of all RLS patients corroborate the diagnosis. Newly, the absence of severe daytime sleepiness is also listed as a non-essential diagnostic feature, which is surprising at first sight, but in line with the experience that RLS patients are more likely to complain about other daytime symptoms such as fatigue or concentration problems despite disturbed nighttime sleep.
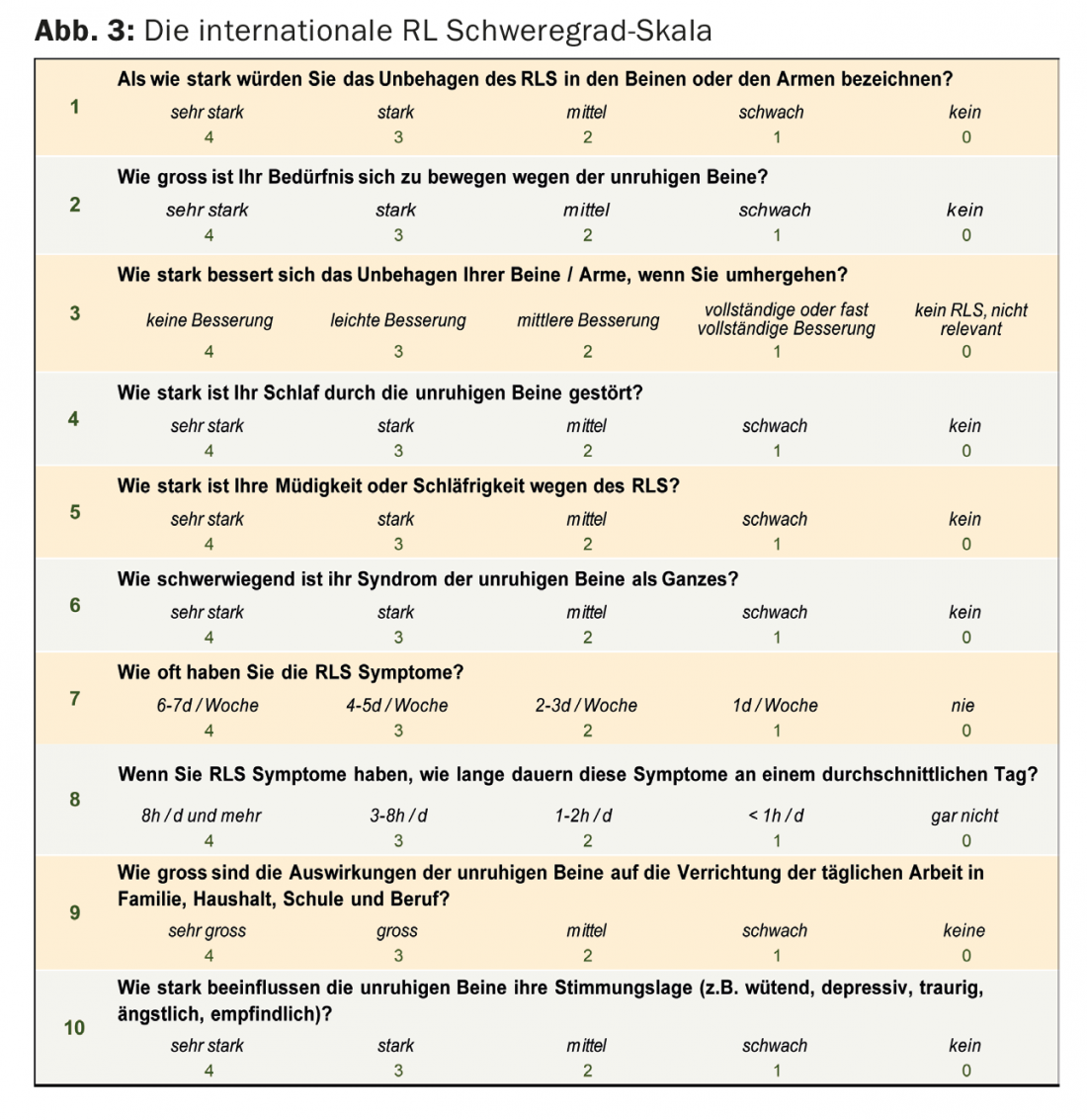
The International RLS Severity Scale (Fig. 3) allows a semiquantitative assessment of RLS symptoms. The immobilization test attempts to objectify the inability to keep the legs motionless in a fixed position; however, the test has not been able to establish itself as a routine diagnostic test.
Differential diagnostics
There are several syndromes that seem to fulfill some or even all of the essential RLS diagnostic criteria, and their delineation sometimes presents some difficulty. These include, but are not limited to, paraesthesia from circumscribed leg injuries, leg cramps, peripheral polyneuropathy, radiculopathy, anxious mood, akathisia, muscle pain, uncomfortable positions, habitual foot bobbing, lower leg edema, or joint inflammation.
Genetics and pathophysiology
Genetic predisposition plays an important role in RLS. Identical twins show higher RLS concordance than fraternal twins. The disease is mostly inherited in an autosomal dominant manner, with high penetrance. Typical for RLS is also the phenomenon of anticipation, i.e. an earlier onset and a more severe course of the disease is observed in the next generation. Genome-wide association studies have identified several gene mutations (PTPRD, BTBD9, MEIS1) associated with increased risk of RLS [2].
The cause of RLS is still largely unclear; evidence for neurodegeneration has not been demonstrated. A central role is played by disturbances in iron and dopamine metabolism. Iron deficiency is a common, treatable cause of RLS, e.g., in pregnancy, pernicious anemia, or renal insufficiency and dialysis. Passenger RLS symptoms may occur postoperatively as a result of blood loss. MRI and postmortem studies showed decreased iron concentrations in the substantia nigra, thalamus, and basal ganglia [3,4]. In addition to the therapeutic response to levodopa, imaging studies demonstrated decreased dopamine D2 receptor density in the striatum and a reduction in dopamine transporter.
Secondary RLS
The most common cause of secondary RLS is diseases associated with iron deficiency. In addition, RLS prevalence is increased in various neurological diseases, such as multiple sclerosis, Huntington’s disease, amyotrophic lateral sclerosis, and Parkinson’s disease. Hypo- and hyperthyroidism and chronic lung disease also appear to be associated with increased risk of RLS. In addition, numerous medications can trigger or exacerbate RLS, especially tricyclic and other antidepressants, lithium, antihistamines, neuroleptics, and alcohol. By definition, the secondary form of RLS disappears after the trigger is removed.
Therapy of RLS
When treating RLS, potentially reversible causes should be addressed before starting drug therapy. This includes iron, vitamin B12, or folic acid supplementation, as well as identifying and discontinuing (if possible) medications that may trigger RLS. Because daytime sleepiness can worsen the intensity of RLS, maintaining good sleep hygiene is also important. Increased caffeine and alcohol consumption is not recommended. Several substance classes are distinguished in pharmacotherapy:
- Levodopa and dopamine agonists
- A2δ-ligands
- Opioids
- Benzodiazepines
In dopaminergic therapy, dopamine agonists are now preferred to levodopa because of the lower risk of augmentation (see box “Augmentation”). The following two dopamine agonists are most commonly used: pramipexole (Sifrol®), starting at 0.125 mg in the evening and gradually increasing to 0.5 mg or 0.75 mg; rotigotine (Neupro®) patches, 1-3 mg/24h. Common side effects include nausea, dizziness, orthostatic hypotension, drowsiness, and impulse control disorder. The efficacy and favorable side effect profile of pregabalin, an A2δ-ligand, was demonstrated in a carefully randomized, double-blind study [5]. The initial dose is 50-75 mg and is typically increased to 300 mg/d. Side effects include dizziness and drowsiness; risk of augmentation is lowered compared with dopamine agonists. In severe RLS and inadequate response to A2δ ligands and/or dopamine agonists, add-on administration of opioids may be helpful, particularly oxycodone (Oxycontin®) 5-10 mg or dihydrocodeine (Codicontin®) 30-90mg. With opioids, there is a risk of increased central apneas. Finally, benzodiazepines, especially clonazepam (Rivotril®) 0.5-1mg, can also relieve suffering. Unlike the other substances, benzodiazepines act by raising the arousal threshold but without reducing the number of PLMS.

Take-Home Messages
- Restless legs syndrome is one of the most common neurological disorders, with a prevalence of approximately 7%.
- Essential RLS features include the urge to move and its occurrence at rest, exacerbation in the evening and at night, and improvement with exercise.
- Important differential diagnoses include, but are not limited to, paraesthesia from circumscribed leg injuries, leg cramps, peripheral polyneuropathy, radiculopathy, anxious mood, akathisia, muscle pain, uncomfortable positions, habitual foot bobbing, lower leg edema, or joint inflammation.
- The disease is mostly inherited in an autosomal dominant manner, with high penetrance. A central role is played by disturbances in iron and dopamine metabolism.
- Several classes of substances are distinguished in pharmacotherapy: (1) levodopa and dopamine agonists, (2) A2δ ligands, (3) opioids, and (4) benzodiazepines. In dopaminergic therapy, dopamine agonists are now preferred over levodopa because of the lower risk of augmentation.
Literature:
- Ekbom KA: Astenia crurum paraesthetica (irritable legs). Acta Med Scand 1944; 118: 197.
- Jiménez-Jiménez FJ, et al: Genetics of restless legs syndrome: An update. Sleep Med Rev 2018; 39: 108-121.
- Connor JR, et al. : Neuropathological examination suggests impaired brain iron acquisition in restless legs syndrome. Neurology 2003; 61: 304309.
- Godau J, et al: Multiregional brain iron deficiency in restless legs syndrome. Mov Disord 2008; 23: 11841187.
- Allen RP, et al: Comparison of pregabalin with pramipexole for restless legs syndrome. N Engl J Med 2014; 370: 621-631.
- Garcia-Borreguero D, et al: Guidelines for the first-line treatment of restless legs syndrome/Willis-Ekbom disease, prevention and treatment of dopaminergic augmentation: a combined task force of the IRLSSG, EURLSSG, and the RLS-foundation. Sleep Med 2016; 21: 1-11.
InFo NEUROLOGY & PSYCHIATRY 2018; 16(4): 12-15.