Knowledge of molecular markers in leukemias could help identify high-risk patients in the future. In addition, there is an opportunity to develop further targeted therapeutic approaches. Some already approved therapeutics show how worthwhile this path to personalized therapy is.
For those who study the pathogenesis of leukemias, the first thing that usually strikes them is that the process is very heterogeneous, as is the variety of leukemic diseases that result. Primarily, leukemias occur as a result of molecular genetic alterations. Particularly regularly affected are the genes and gene products of, for example, BCR-ABL, p53, DNMT3A, and others. But what diagnostic and, above all, therapeutic role do such gene mutations play?
Gene mutations and chromosomal aberrations occur naturally in every healthy person with increasing age. In the age over 70 years such gene changes can be found in 10-50% of all test persons. Nevertheless, not all of these elderly people develop leukemia. The disease-causing moment in many cases is a clonal accumulation of mutations in hematopoiesis. This means that altered blood cells form a numerically enlarged clone and thus become blood picture-determining. This so-called clonal hematopoiesis (“clonal hematopoiesis of indeterminate potential”, CHIP) is, according to current knowledge, a decisive risk factor for hematological neoplasia. In addition, chromosomal aberrations in the peripheral blood and, in older men, loss of the Y chromosome in the blood cells are considered risk factors for the development of leukemia.
Of gene markers and marker genes
Acute myeloid leukemia (AML) is still often associated with a very poor prognosis. In addition, especially for older patients, there are often no or only a few targeted therapy options. However, genetic targets (e.g. FLT3, IDH1/2, BCL2) now make it possible to better classify patients and their respective prognoses (favorable, intermediate, poor) and also to treat them with the aid of the first targeted therapeutics.
A not infrequently occurring genetic alteration is the FLT3 mutation. Approximately 25% of AML patients have one or more alleles for this gene. The mutation is considered unstable and thus causes different therapeutic sensitivities, which may well play an important role for future treatment regimens. Nevertheless, the world’s first genotype-specific phase III trial called RATIFY [1] in patients with AML and FLT3 mutation showed that midostaurin, a multitarget tyrosine kinase inhibitor, in combination with chemotherapy increased survival. Event-free survival additionally increased from 3.2 to 8.2 months. Thus, after more than 30 years, the now approved midostaurin is considered a real breakthrough in AML therapy.
A “blockbuster” future therapeutic option in AML is venetoclax, a drug that acts as a stem cell target for BCL2 and is currently in Phase III of potential approval. Even in monotherapy, studies to date have shown good response rates (20% ORR) with venetoclax in patients with R/R AML. In addition, 70% of responders lived longer than twelve months. Response to venetoclax was rapid and durable with few recurrences.
Similar to AML, acute lymphoblastic leukemia (ALL) is also very heterogeneous from a molecular point of view (more than ten different subgroups), which is why the standard chemotherapy based on the “one fits all” approach cannot really be helpful. There will certainly be more movement in the treatment of these tumor entities in the future, e.g. in the search for suitable TKIs as well as, above all, approaches from immunotherapy or CAR-T cell therapy. With blinatumomab (anti-CD19) and inotuzumab ozogamicin (anti-CD22), the first approved therapeutics are already on the market.
After survival comes quality of life
Following the introduction of TKIs into chronic myeloid leukemia (CML) therapy, the 10-year survival rate for this disease is now up to 83%, meaning that survival with CML is similar to that of the general population. However, this means that for patients with CML it is no longer just a matter of survival. Likewise, progression-free survival, the side effect profile, long-term toxicity, and especially quality of life play a major role for them. For oncologists, however, the focus is on the opportunity to achieve a therapy-free remission in CML patients with medication, because this effectively leads to a better quality of life, fewer side effects and less long-term toxicity.
Currently, five original drugs exist worldwide, of which imatinib, which has also been approved in this country, is certainly one of the well-known “highlights” of modern CML treatment. Thus, imatinib showed a 5-year survival in CML of more than 95%. However, additional cytogenetic (chromosomal) aberrations may reduce the success of therapy. Therefore, bone marrow aspiration is still recommended in addition to PCR to detect alterations in CML patients.
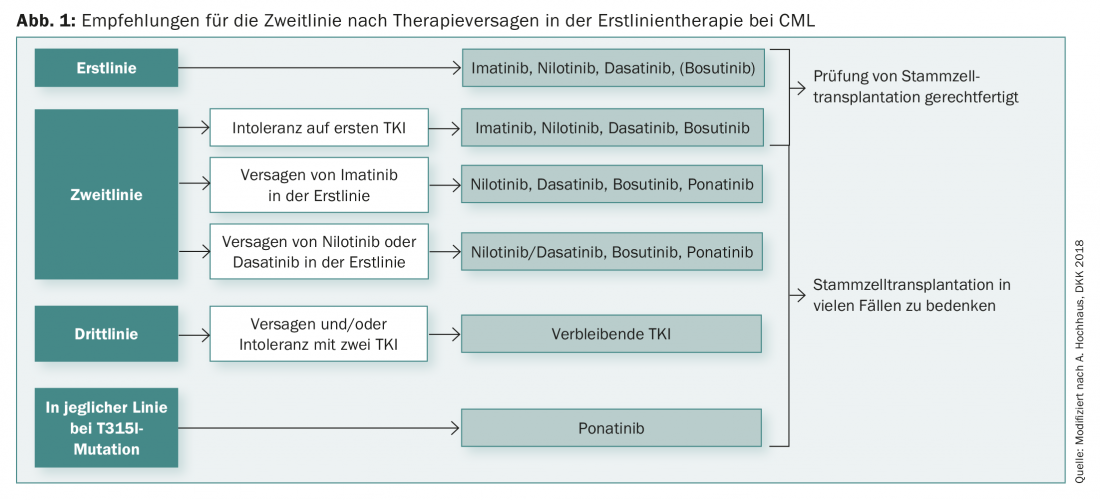
However, the choice of first-line therapy in CML does not depend on genetics alone. In addition, individual therapy goals should play a role, as should any concomitant diseases (comorbidities). For many patients, the question of the deductibility of the therapy is also very important. There are also initial recommendations in this regard, which, according to current studies, allow therapy to be discontinued at deep molecular remission above 18 months in order to reduce side effects, for example.
The duration of deep molecular remission (for at least two years) is then also a crucial factor for relapse prevention. However, it is true in many cases that therapy is possible again even after a relapse and that the patient can achieve remission again under therapy. After failure of first-line therapy, there are now a number of diverse second-line options for CML (Fig. 1).
Source: 33rd German Cancer Congress, February 21-24, 2018, Berlin.
Literature:
- Stone RM, et al: N Engl J Med 2017; 377: 454-464.











