The SEQUENCE comparative study recently published in the New England Journal of Medicine (NEJM) compared the two interleukin (IL)-23 and IL-12/-23 inhibitors risankizumab (SKYRIZI®) and ustekinumab in Crohn’s disease (CD) patients [1-3]. Supplementary post-hoc analyses have now been presented at this year’s United European Gastroenterology Week (UEGW) from October 12 to 15, 2024 in Vienna [4-6]. The results show, among other things, that risankizumab achieves numerically higher efficacy rates compared to ustekinumab regardless of disease duration, leads to greater normalization of biomarkers and significantly improves quality of life [4-6].
In the SEQUENCE study, over 500 CD patients with treatment failure were randomized to one or more TNF inhibitors and treated open-label for 48 weeks with either risankizumab (N=255) or ustekinumab (N=265) [1]. Both primary endpoints were achieved: With clinical remission after 24 weeks (CDAI < 150) Risankizumab was non-inferior to ustekinumab (58.6 % vs. 39.5 %. With regard to the second primary endpoint, endoscopic remission (SES-CD ≤ 4 and ≥ 2 points lower than baseline) after 48 weeks, risankizumab was significantly superior to ustekinumab (31.8 % vs. 16.2 %, p < 0.001) [1]. In addition, the overall rate of treatment-emergent adverse events (TEAEs) was low in both groups (27.9% vs. 21.9%) and no new safety signals were identified with risankizumab compared to the pivotal trials [1, 7, 8]. Supplementary post-hoc analyses of the comparative study have now been presented at UEGW 2024.
Numerically higher efficacy rates with risankizumab independent of disease duration with greatest benefit after starting treatment as early as possible [4]
Early treatment with advanced therapies such as biologics can slow or interrupt the progression of CD and thus improve patients’ quality of life and prevent further complications such as fistulas, hospitalization and surgery [9, 10]. The efficacy of risankizumab compared to ustekinumab in patients with different disease durations (<2 Jahre, 2-5 Jahre, 5-10 Jahre,>10 years) has now been analyzed in a post-hoc analysis of the SEQUENCE study [4]. The result: Regardless of how long the patients had had the disease, a numerically larger proportion achieved clinical and endoscopic remission after 24 and 48 weeks as well as endoscopic response and steroid-free clinical and endoscopic remission after 48 weeks with risankizumab [4]. Particularly in the endoscopic response at 48 weeks, more risankizumab patients reached the endpoint* compared to the ustekinumab group( <2 years: 52.8 % vs. 25.6 %, P < 0.05; 2-5 years: 40.7 % vs. 12.9 %, P < 0.001; 5-10 years: 46.3 % vs. 25.4 %, P < 0.05;>10 years: 44.1 % vs. 23.6 %, P < 0.01). Patients with risankizumab and a disease duration of ≤2 years benefited the most, which supports early intervention in CD patients [4].

Fig 1: Higher normalization rates of the biomarkers hs-CRP and FC with risankizumab. Post-hoc analysis, all p-values are nominal and not multiplicity controlled. FC = fecal calprotectin; hs-CRP = high-sensitivity C-reactive protein; Q8W = every 8 weeks; RZB = risankizumab; s.c. = subcutaneous; UST = ustekinumab. Adapted from [5]
Biomarkers underline superiority of risankizumab compared to ustekinumab [5]
In the primary data analysis of the SEQUENCE study, patients with risankizumab showed a greater reduction in fecal calprotectin (FC) and C-reactive protein (CRP) than with ustekinumab [1]. FC and CRP are the two most commonly used biomarkers in CD and serve as objective markers of intestinal inflammation [11]. Normalization of FC and CRP is therefore also recommended as a medium-term treatment goal in the STRIDE II guidelines [11]. A post-hoc analysis of the SEQUENCE study presented at the UEGW shows that this goal is achievable for more patients on risankizumab than on ustekinumab: here, a larger proportion of risankizumab patients with elevated FC (>1.5 %) achieved normalized FC (>1.5 %):In with elevated FC (>250 mg/kg) or CRP (>5 mg/L) at baseline achieved normalization of CRP at weeks 24 and 48 and normalization of FC at weeks 8, 24 and 48 (nominal p < 0.01) (Fig. 1) [5]. In addition, more risankizumab patients achieved normalization of biomarkers in combination with clinical remission and endoscopic response than patients receiving ustekinumab (Fig. 2) [5].
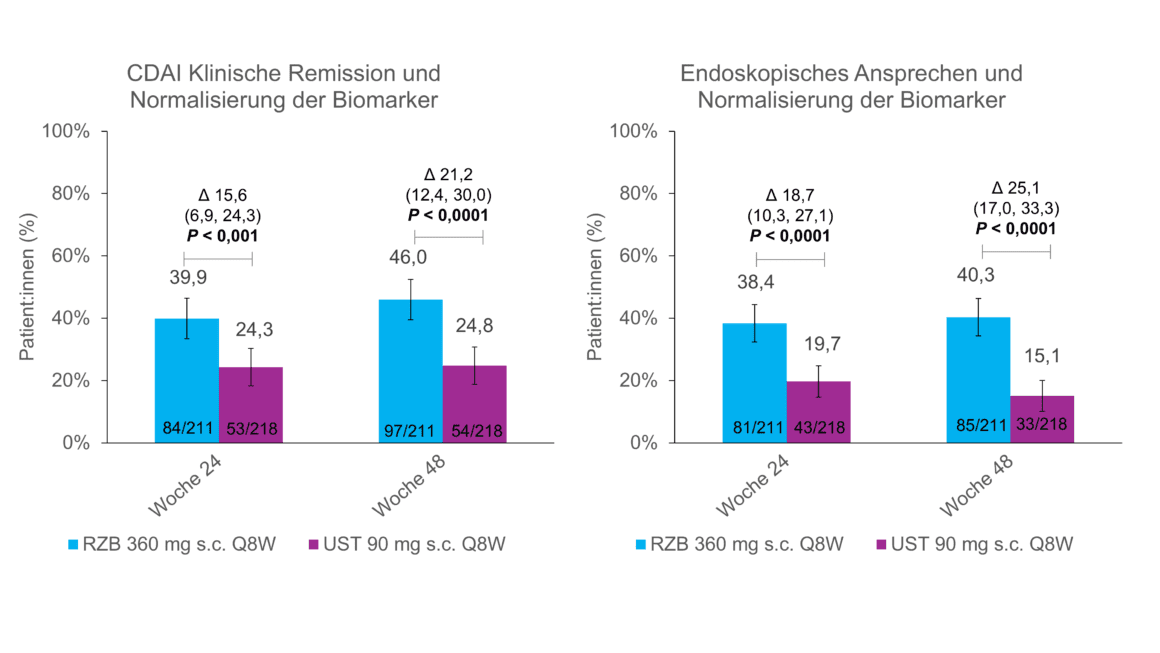
Fig. 2: Clinical remission or endoscopic response + normalization of biomarkers more frequent with risankizumab. Post-hoc analysis, all p-values are nominal and not multiplicity controlled. CDAI = Crohn’s Disease Activity Index; Q8W = every 8 weeks; RZB = risankizumab; s.c. = subcutaneous; UST = ustekinumab. Adapted from [5]
Improvement in quality of life and psychological symptoms with risankizumab [6]
Another post-hoc analysis looked at the improvement in quality of life and analyzed various patient-reported outcomes (PROs) such as IBDQ and SF-36v2 [6]. A significantly larger proportion of patients treated with risankizumab showed clinically meaningful improvements in IBDQ remission (week 24: 52.5% vs. 30.9%; week 48: 49.8% vs. 33.2%) and improvement in physical and mental SF-36v2 compared to the ustekinumab group [6]. In addition, a significantly lower proportion of patients in the risankizumab group reported symptoms such as fatigue, depression or anxiety at weeks 24 and 48 compared to the ustekinumab patients [6].
Conclusion
The SEQUENCE study is the first head-to-head study in CD to show superiority of a biologic over another biologic [1]. The in-depth post-hoc analyses presented here now also underline the improved efficacy of risankizumab in patients with different disease durations, biomarkers and quality of life [4-6]. These results highlight the potential benefit of early intervention with risankizumab to further improve patients’ disease control and quality of life.
*Post-hoc analysis, all p-values are nominal and not multiplicity controlled.
Abbreviations: CDAI = Crohn’s Disease Activity Index; IBDQ = Inflammatory Bowel Disease Questionnaire; PRO = Patient-reported outcome; SES-CD = Simple Endoscopic Score for Crohn’s Disease; SF-36v2 = Short Form-36version 2; TEAE = Treatment-emergent adverse events.
Source:
United European Gastroenterology Week (UEGW) 2024, October 12-15, 2024, Vienna (Austria).
Report: Dr. sc. nat. Stefanie Jovanovic
Short technical information SKYRIZI®

CH-SKZG-240100 11/2024
This article was produced with the financial support of AbbVie AG, Alte Steinhauserstrasse 14, Cham.
This article has been released in German.
Literature
1 Peyrin-Biroulet, L., et al, Risankizumab versus ustekinumab for moderate-to-severe Crohn’s disease. N Engl J Med, 2024. 391(3): p. 213-223.
2. current expert information on SKYRIZI® (risankizumab) Crohn’s disease at www.swissmedicinfo.ch.
3. current technical information on ustekinumab at www.swissmedicinfo.ch.
4 Peyrin-Biroulet, L., et al. Efficacy of Risankizumab Versus Ustekinumab by Duration of Disease in Patients With Moderate to Severe Crohn’s Disease: A Posthoc Analysis From the Phase 3 SEQUENCE Study. PP0589. Poster presented at UEGW; Vienna, Oct 12-15, 2024.
5 Colombel JF, et al: Inflammatory Biomarker Reduction and Improvement in Clinical and Endoscopic Outcomes With Risankizumab Versus Ustekinumab in Patients With Moderate to Severe Crohn’s Disease: A Posthoc Analysis From the Phase 3 SEQUENCE Study. PP0588. Poster presented at UEGW; Vienna, Oct 12-15, 2024.
6 Loftus EV, et al: Improvement in Health-Related Quality of Life in Patients With Moderate to Severe Crohn’s Disease Treated With Risankizumab Versus Ustekinumab in the Phase 3B SEQUENCE Study. MP677. Poster presented at UEGW; Vienna, Oct 12-15, 2024.
7 D’Haens, G., et al, Risankizumab as induction therapy for Crohn’s disease: results from the phase 3 ADVANCE and MOTIVATE induction trials. Lancet, 2022. 399(10340): p. 2015-2030.
8 Ferrante, M., et al, Risankizumab as maintenance therapy for moderately to severely active Crohn’s disease: results from the multicentre, randomized, double-blind, placebo-controlled, withdrawal phase 3 FORTIFY maintenance trial.Lancet, 2022. 399(10340): p. 2031-2046.
9 Danese, S., G. Fiorino, and L. Peyrin-Biroulet, Early intervention in Crohn’s disease: towards disease modification trials. Gut, 2017. 66(12): p. 2179-2187.
10 Ben-Horin, S., et al, Efficacy of Biologic Drugs in Short-Duration Versus Long-Duration Inflammatory Bowel Disease: A Systematic Review and an Individual-Patient Data Meta-Analysis of Randomized Controlled Trials. Gastroenterology, 2022. 162(2): p. 482-494.
11 Turner, D., et al, STRIDE-II: An Update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) Initiative of the International Organization for the Study of IBD (IOIBD): Determining Therapeutic Goals for Treat-to-Target strategies in IBD. Gastroenterology, 2021. 160(5): p. 1570-1583.
The references can be requested by specialists at medinfo.ch@abbvie.com.











