5α-Reductase inhibitors are used to reduce prostate volume and associated symptoms. Possible side effects include sexual dysfunction and depressive disorders. Neuroactive steroids are thought to play an important role in this, but the exact mechanisms are not yet clear. Animal studies showed relevant changes in the dopaminergic system, among others, after treatment with finasteride. Depression, especially anhedonia, is associated with dysfunction of dopaminergic processes.
The prevalence of benign prostatic hyperplasia (BPH) in men aged 50-70 years is 50-75% and approximately 80% in those over 70 years, respectively [1]. The therapy depends on the symptoms and the stage of the disease. Drug treatment options include phytotherapeutics (e.g., saw palmetto extracts) and selective α-blockers, antiandrogens, or 5α-reductase inhibitors (5-ARIs). The effects of 5-ARIs (e.g., finasteride) are based on a sharp decrease in 5α-dihydrotestosterone (DHT) concentration due to irreversible blockade of 5α-reductase in sexual organs, brain, skin, and other organs and tissues [2]. This has the effect of preventing the conversion of testosterone to the more potent androgen dihydrotestosterone and blocking neuroactive depressant steroids (e.g., allopregnanolone, androsterone) [2]. 5-ARIs should be taken for at least 6-12 months. In addition to desirable therapeutic effects such as reduction in prostate size and improvement in micturition symptoms, there may be accompanying symptoms such as sexual dysfunction and depressive disorders. In a secondary analysis, Saengmearnuparp et al. examined the current state of knowledge regarding the association of 5-ARI and depression (box) . The following is an excerpt of the results.

Dose- and time-dependent changes in animal models.
As shown by experimental studies in rodents, dysfunctions of the dopaminergic system and modifications of the hypothalamic-pituitary-adrenal axis are among the changes triggered by 5-ARIs [4,5]. In contrast to the acute effects, finasteride elicited dose- and time-dependent behavioral modification in adult male Wistar rats from 24 h to 7 days of treatment and treatment for ≥14 days in animal experiments [4,5]. The results of studies in C57BL/6N mice showed that brain DHT levels were significantly decreased in a group treated with finasteride for 24 h to 7 days, but brain testosterone levels did not change [6].
In male Sprague-Dawley rats, ≥14 days of low-dose finasteride treatment had no significant effects on the concentration of 5α-reductase products such as dihydroprogesterone (DHP), tetrahydroprogesterone (THP), and dihydrotestosterone (DHT), indicating compensatory processes [7]. In contrast, plasma DHT levels in the finasteride-treated group were significantly reduced [7].
Animal findings on dopaminergic processes.
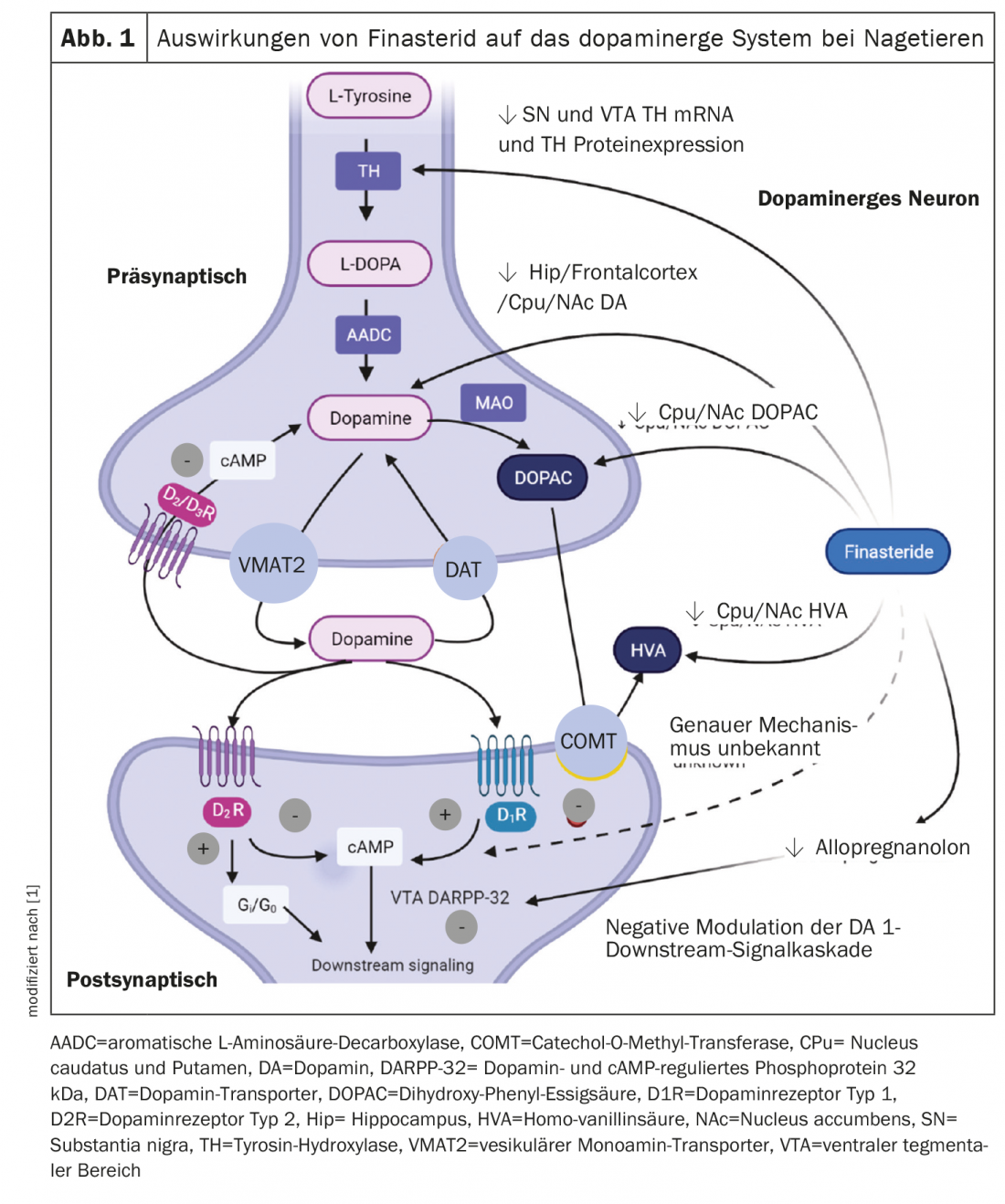
Prolonged administration of finasteride decreased dopamine concentration and its metabolites in different brain regions in a rat model [8]. These results were observed only in the finasteride group during adolescence or the period from testosterone surge. The levels of dopamine, dihydroxyphenylacetic acid, and homovanillic acid in the caudate nucleus, putamen, and nucleus accumbens were also significantly decreased. In addition, a reduction in mRNA and protein expression of tyrosine hydroxylase was observed in the substantia nigra and ventral tegmental areas of these rats. The effects of finasteride on the dopaminergic system observed in animal experiments are summarized in Figure 1 [8]. Overall, the results suggest that a decrease in DHT, the major androgenic metabolite, during a period of testosterone surge may be one of the causes of the changes in the dopaminergic system. It has also been documented that alterations in other neuroactive steroids such as testosterone, estrogen, and glucocorticoid, via modulation of dopaminergic signaling in adolescent rats pose a risk for dopaminergic signal transduction in adolescent rats [9].
Hippocampus: decreased neurogenesis and increase in neuroinflammation.
Another mechanism to explain depressive symptoms after treatment with 5-ARIs is the reduction of neurogenesis in the hippocampus. Furthermore, finasteride treatment was associated with an increase in proinflammatory cytokines in the hippocampal region [12,13]. According to preclinical and clinical data, there is an association between neuroinflammation and depressive behavioral tendencies [10,11].
Based on the above and other studies, the authors deduce the following: Neuroinflammation due to finasteride administration causes a reduction in neurogenesis and subsequently leads to depressive symptoms. Finasteride-induced neuroinflammation alters dopaminergic processes and serotonin synthesis, which promotes depressive behavior [14]. In addition, the authors mention that there is evidence that finasteride promotes intestinal dysbiosis, thereby affecting systemic inflammation. Thus, finasteride resulted in an increase in the Bacteroidetes strain in the intestine when treated for 24 h to 7 days and for ≥14 days. Gut microbiota dysbiosis has been associated with depression-like behaviors in rats [8,14].
Literature:
- Saengmearnuparp T, et al: The connection of 5-alpha reductase inhibitors to the development of depression. Biomed Pharmacother 2021; 143: 112100. doi: 10.1016/j.biopha.2021.
- “Hair growth with consequences: Successful Finasteride Treatment May Lead to Permanent Problems,” www.deutsche-apotheker-zeitung.de/daz-az/2018/daz-16-2018/haarwuchs-mit-folgen, (last accessed Sept. 05, 2022).
- Traish AM: Post-finasteride syndrome: a surmountable challenge for clinicians. Fertil Steril 2020; 113 (1): 21-50.
- Sasibhushana RB, et al: Repeated finasteride administration induces depression-like behavior in adult male rats. Behav Brain Res 2019; 365: 185-189.
- Li L, et al: Finasteride inhibited brain dopaminergic system and open-field behaviors in adolescent male rats, CNS Neurosci Ther 2018; 24 (2): 115-125.
- Römer B, et al: Finasteride treatment inhibits adult hippocampal neurogenesis in male mice, Pharmacopsychiatry 2010; 43 (5): 174-178.
- Giatti S, et al: Effects of subchronic finasteride treatment and withdrawal on neuroactive steroid levels and their receptors in the male rat brain. Neuroendocrinology 2016; 103 (6): 746-757.
- Yu M, et al: Variations in gut microbiota and fecal metabolic phenotype associated with depression by 16S rRNA gene sequencing and LC/MS-based metabolomics. J Pharm Biomed Anal 2017; 138: 231-239.
- Sinclair D, et al: Impacts of stress and sex hormones on dopamine neurotransmission in the adolescent brain. Psychopharmacology 2014; 231 (8): 1581-1599.
- Snyder JS, et al: Adult hippocampal neurogenesis buffers stress responses and depressive behavior, Nature 2011; 476 (7361): 458-461.
- Malberg JE, et al: Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus, J. Neurosci 2000; 20 (24): 9104-9110.
- Diviccaro S, et al: Treatment of male rats with finasteride, an inhibitor of 5alpha-reductase enzyme, induces long-lasting effects on depressive-like behavior, hippocampal neurogenesis, neuroinflammation and gut microbiota composition. Psychoneuroendocrinology 2019; 99: 206-215.
- Felger JC, Treadway MT: Inflammation effects on motivation and motor activity: role of dopamine, Neuropsychopharmacology 2017; 42 (1): 216-241.
- Troubat R, et al: Neuroinflammation and depression: a review, Eur J Neurosci 2021; 53 (1): 151-171.
GP PRACTICE 2022: 17(9): 26-27