Acute symptomatic post-stroke seizures generally do not require long-term therapy and the current recommended time limit in guidelines to differentiate epilepsy after stroke is 7 days. Validated tools, such as the SeLECT model, have been developed to more accurately assess the risk of recurrence of epileptic seizures after stroke and are available digitally for use at the patient’s bedside.
You can take the CME test in our learning platform after recommended review of the materials. Please click on the following button:
One person in 20 in Europe will suffer an epileptic seizure during their lifetime. This makes epileptic seizures one of the most common neurological disorders. First-time seizures occur particularly frequently in infancy and childhood, while there is a plateau in incidence in young adulthood. However, epileptic seizures are even more common over the age of 55, after which the incidence increases steadily. The reason for this is the ageing of the brain and the accumulation of various brain diseases.
Stroke is the most common cause of epilepsy with first manifestation in adulthood in Europe. In people over 65, half of epilepsies are due to cerebrovascular disease. Post-stroke seizures are not only frequent, but also relevant. They are associated with higher mortality, disability and a risk of further cognitive deficits [1].
Practical approach
If a suspected seizure occurs after a stroke, three primary questions should be clarified:
- Is it an epileptic seizure?
- Do you have epilepsy?
- What treatment is appropriate in this situation?
1. is it an epileptic seizure?
The most important differential diagnoses (mimics) of epileptic seizures in this age group are transient ischemic attacks (TIA), transient focal neurological episodes in amyloid angiopathy (TFNE, also known as amyloid spells ), syncope and other autonomic phenomena. Functional or dissociative seizures and migraines are rarer in this age group.
Epileptic seizures are usually short episodes lasting around 30 seconds to 2 minutes, which are often accompanied by positive symptoms such as visual auras, tingling paresthesia, myoclonus or other motor phenomena. In TIAs and TFNE, on the other hand, neurological deficits such as paresis, visual field defects or speech disorders are in the foreground. Disturbances of consciousness are typical of epileptic seizures, but rarely occur in the differential diagnoses mentioned. A duration of more than 15 minutes is atypical for epileptic seizures and is more indicative of TIAs, TFNE or other neurological disorders, such as a migraine aura.
However, it should be noted that epileptic seizures in older people are often oligosymptomatic. A typical manifestation is a focal, unconsciously experienced seizure characterized by pausing and staring. Such a seizure can easily be overlooked and requires close observation and targeted addressing of the affected person during the seizure. Postictal impairments such as confusion often occur afterwards.
The diagnostic differentiation is usually based on a thorough medical history and external observation, but is often difficult. The detection of epilepsy-typical changes in the interictal EEG, the recording of an ictal video EEG and the differentiation of differential diagnoses using magnetic resonance imaging can be helpful. The sensitivity of a short routine interictal EEG to detect epilepsy-typical potentials is only 20-30% and is not sufficient to rule out epilepsy in the case of negative findings. The sensitivity can be increased to up to 70% by early recording, repeated EEGs and performing a long-term EEG overnight.
2 Is epilepsy present?
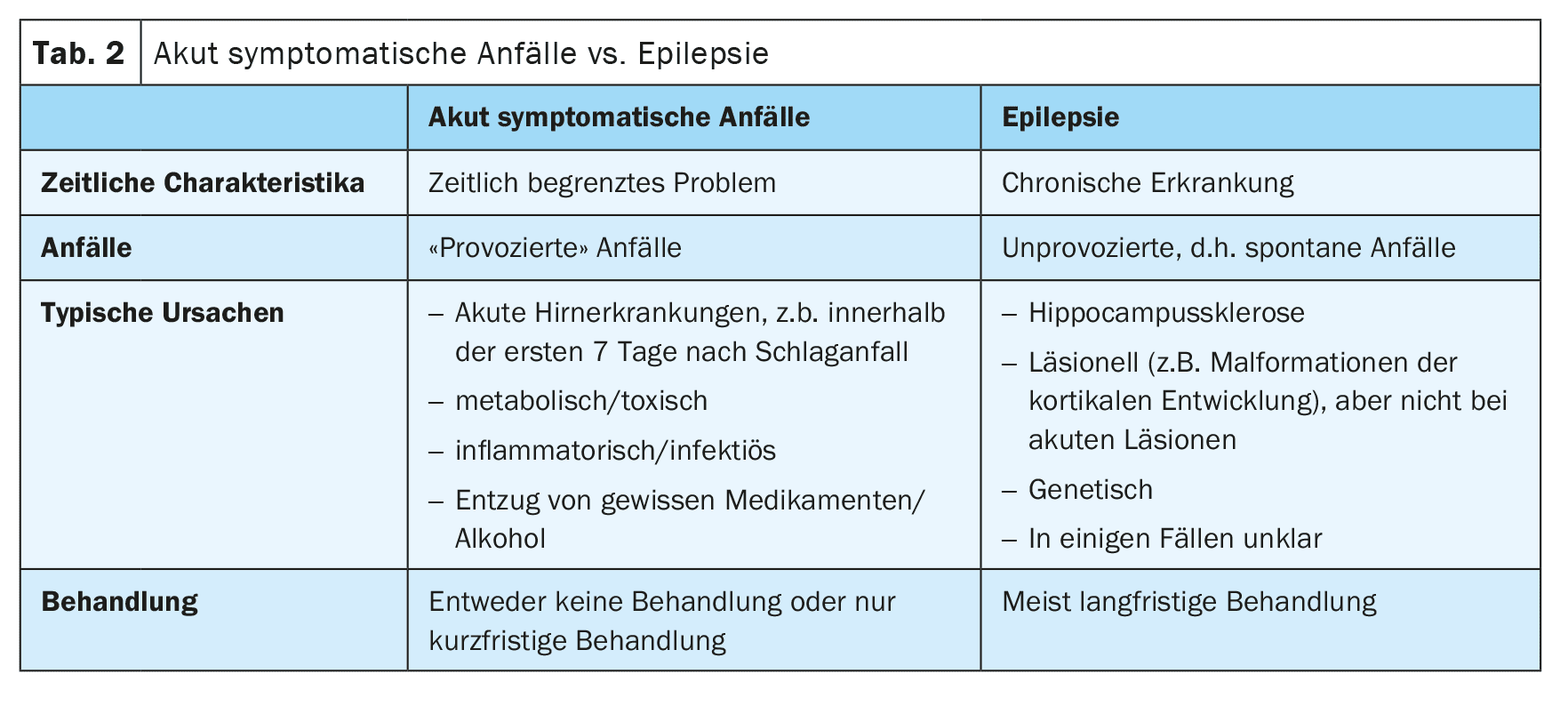
If it is clear that the seizure is epileptic, it must be clarified whether the seizure was provoked or unprovoked (Table 1). The motto is: “Everyone with epilepsy has seizures, but not everyone with seizures has epilepsy.”
Today, we refer to provoked seizures as “acute symptomatic seizures” [2]. These occur in close temporal connection with a brain disease or metabolic, toxic or inflammatory disorder. Epileptic seizures that occur in the first 7 days after a stroke are considered acute symptomatic. The reason for this is that these seizures are triggered by acute toxic and inflammatory changes in the context of the stroke and are not an expression of underlying epilepsy. Accordingly, the risk of subsequent unprovoked seizures is rather low. However, the 7-day limit is not well established, and recent unpublished data suggest that seizures within the first month are also associated with a low risk of subsequent unprovoked seizures and could therefore also be considered acutely symptomatic (Table 2).
In contrast, seizures that occur at a later time after a stroke are considered unprovoked unless they were triggered by other factors, such as hyponatremia. If an unprovoked seizure was probably caused by the underlying stroke, i.e. has a matching semiology, a high recurrence risk of over 60% for further unprovoked seizures within the next 10 years can be assumed. For this reason, according to the current guidelines, a diagnosis of structural epilepsy can be made after a first unprovoked epileptic seizure following a stroke [3].
3. what treatment is appropriate in this situation?
The distinction between acute symptomatic seizures and structural epilepsy is crucial, as the treatment approaches are very different. A detailed description of the treatment of these two entities follows later in this article.
In summary, the guidelines recommend no or only short-term treatment of acute symptomatic seizures, while epilepsy usually requires long-term treatment (Fig. 1) [4]. If treatment is started after an acute symptomatic seizure, it should be relatively aggressive and with a fast-acting drug. The reason for this is that the risk of seizures is highest in the first few days after a stroke and decreases rapidly thereafter. In contrast, the treatment of epilepsy in older people follows the principle of start low, go slow, as the clearance of antiepileptic drugs (ASMs) is reduced in old age.
Risks after acute symptomatic seizures
About 40% to 50% of first-time epileptic seizures are acutely symptomatic. Cerebrovascular disease is one of the most common causes of acute symptomatic seizures, particularly in older people. Seizures are particularly common in patients treated in intensive care units following a stroke, with approximately 20-30% of these patients experiencing seizures. However, many of these seizures are non-convulsive, show only mild symptoms or are completely subclinical.
If continuous EEG monitoring is carried out, more seizures can be detected. In a prospective study after intracerebral hemorrhage, subclinical seizures were detected in almost half of the patients [5]. It remains unclear whether subclinical seizures have a similar significance to clinical seizures. However, there is evidence that subclinical seizures are associated with a similarly high risk of later development of epilepsy and poor outcome as clinical seizures. Therefore, the treatment of subclinical seizures should probably be similar to the treatment of clinical seizures.
Risk of developing epilepsy: Acute symptomatic seizures are not synonymous with epilepsy as they are triggered by acute brain damage. However, they indicate that the affected person may have a low seizure threshold and that the stroke has activated a potentially epileptic network that may later contribute to the development of epilepsy. Acute symptomatic seizures are therefore the most important risk factor for the development of epilepsy after a stroke.
In people who have suffered an acute symptomatic seizure, the risk of later epilepsy is around 20-40%. The highest risk of over 80% is observed in patients who have had an acute symptomatic status epilepticus. An increased risk of around 60-70% is also likely to exist in patients with a tonic-clonic acute symptomatic seizure on the day of the stroke.
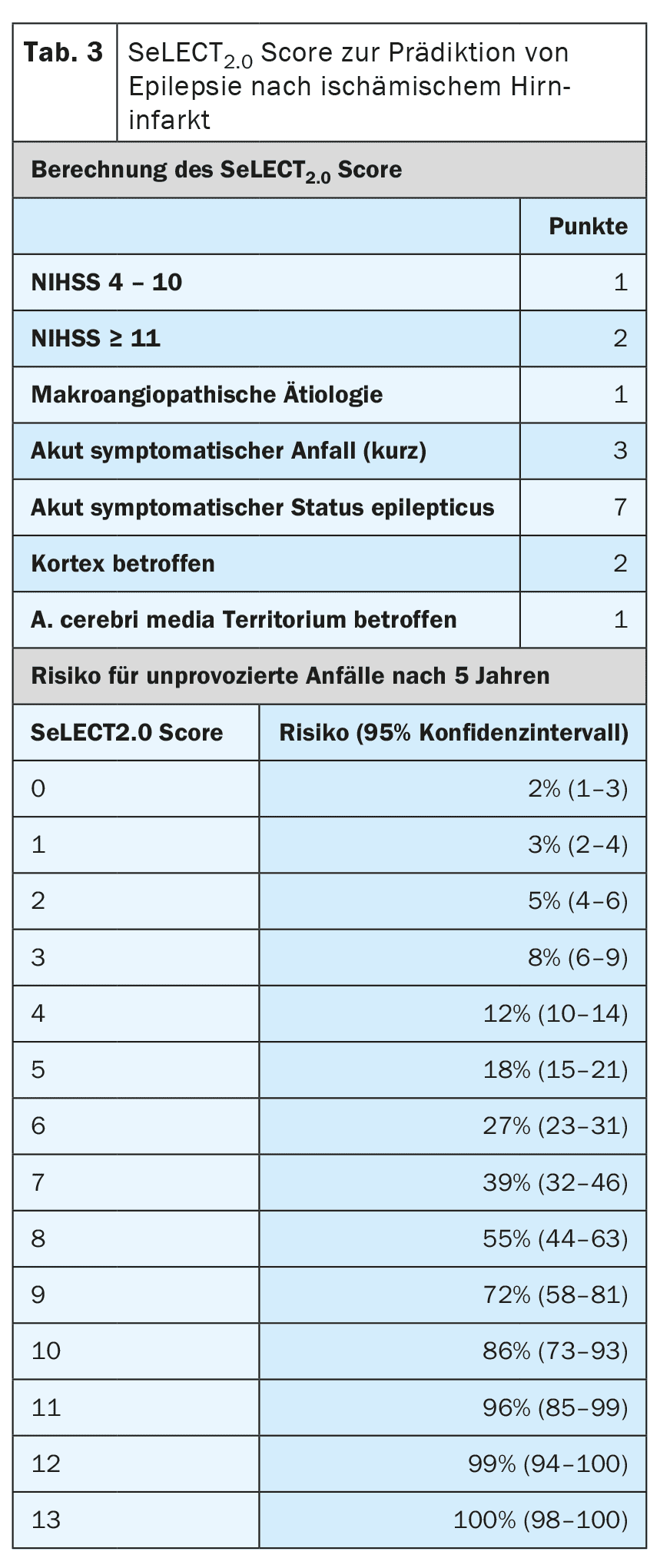
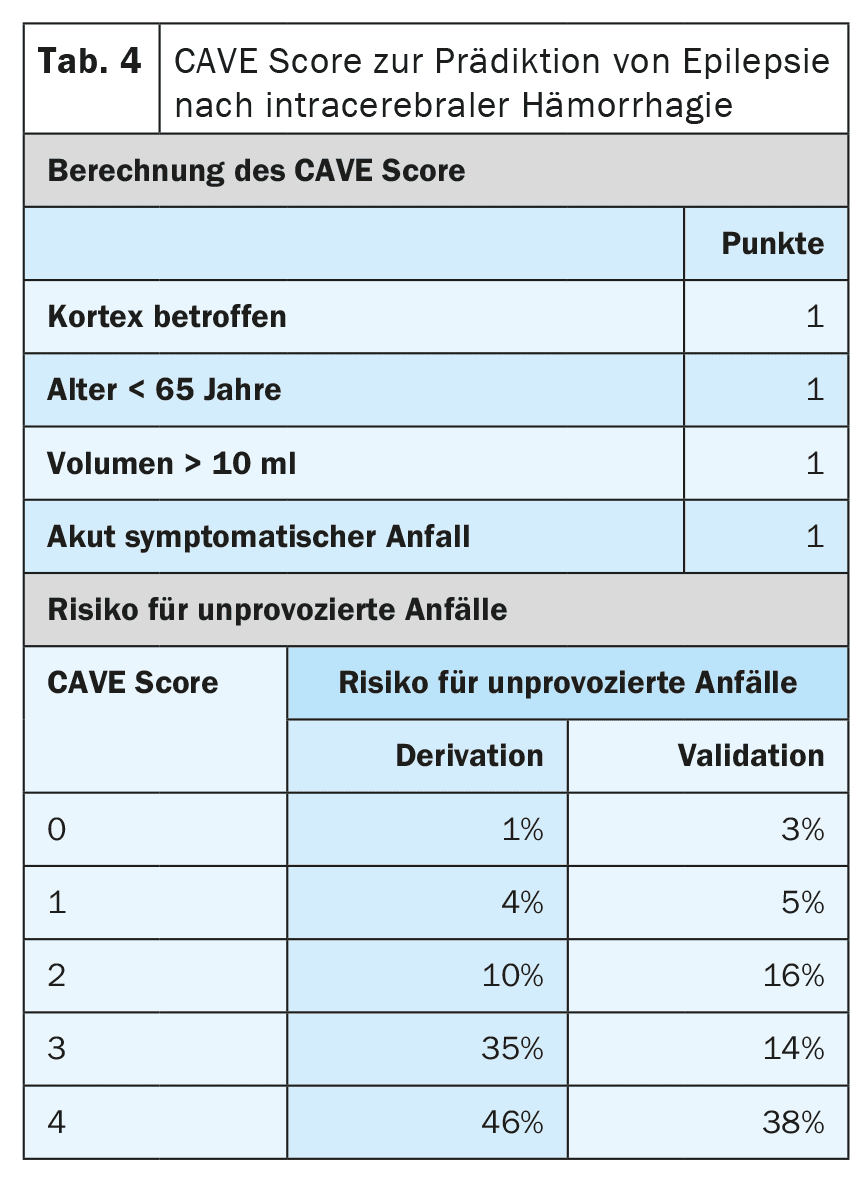
Other risk factors for the development of epilepsy after a stroke are the type, location, severity and aetiology of the infarction. There are now very well validated prognostic models that can predict the risk with a high degree of accuracy. The SeLECT model (Table 3) [6,7] is recommended for ischemic cerebral infarctions, while the CAVE model (Table 4) is recommended for hemorrhagic infarctions [8]. A smartphone app is available for the SeLECT model under the name SeLECT Score , which facilitates the calculation at the patient’s bedside.
Impact on outcome: Post-stroke seizures are associated with poorer outcome, including higher mortality, greater disability and an increased risk of cognitive impairment, including dementia [20]. These effects are much more pronounced in acute symptomatic seizures compared to later unprovoked seizures. The worst outcome is after acute symptomatic status epilepticus, which increases the risk of mortality tenfold. In most cases, however, the reason for death in acute symptomatic status epilepticus is withdrawal of treatment or a palliative approach.
It is important to emphasize that these correlations represent association and not necessarily causation. In other words, seizures after a stroke are an indicator of particularly severe brain damage caused by the infarction. Whether seizure suppressive treatment improves the outcome is questionable. Most studies tend to indicate that the outcome is not improved.
Treatment of acute symptomatic seizures
There is little robust evidence for the treatment of acutely symptomatic seizures, but many uncertainties and misunderstandings. The current DGN guideline primarily recommends that acutely symptomatic seizures should not be treated [4]. The main reason given for this is that frequent overtreatment should be avoided. If treatment is nevertheless started after individual assessment, it should only be carried out in the short term and during the acute phase, i.e. in the first 7 to 14 days after the stroke. It is also recommended to use prognostic models such as the SeLECT or CAVE score for individual assessment.
However, the reality differs considerably from the guideline recommendations. Prognostic registries from Germany and the USA as well as international surveys show that 9 out of 10 patients with acute symptomatic seizures are treated with ASMs. About half of these patients receive treatment for several months, and up to a third are treated with ASMs on a long-term basis. Most of this data comes from specialized centers, so the actual numbers in the general population may be even higher. There is therefore a clear discrepancy between the guideline recommendations and clinical practice.
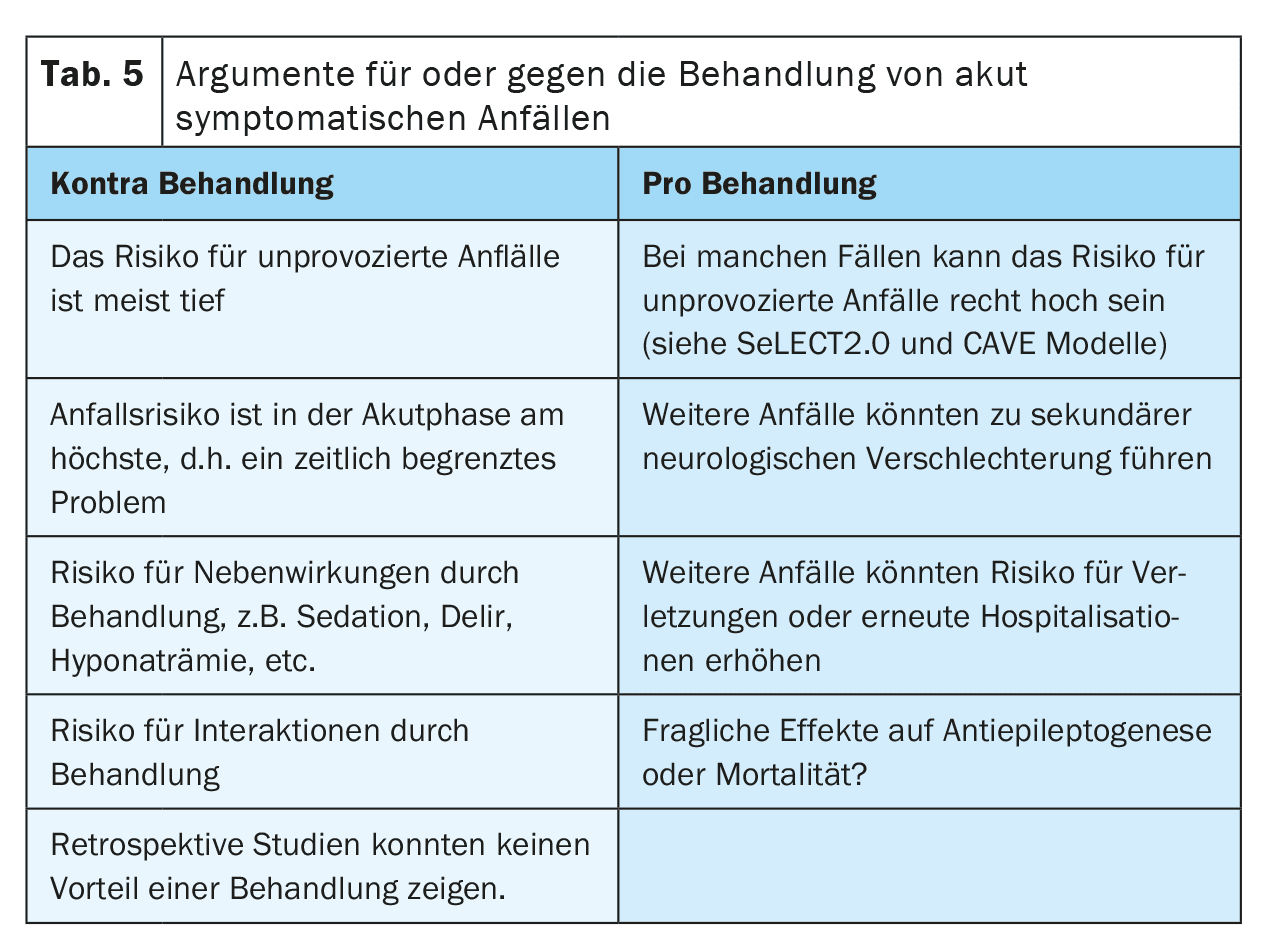
There are several arguments that could speak for or against the treatment of acute symptomatic seizures with ASMs (Table 5). One argument in favor of treatment is the fear of further seizures that could lead to falls or injuries or cause distress to the patient. It is also debated whether seizures could cause secondary deterioration after a stroke due to the transient increased need for perfusion and metabolism. This is particularly relevant in patients with high-grade vascular stenosis, vasospasms, increased intracranial pressure or after neurosurgery.
On the other hand, treatment with ASMs can also have side effects. For example, psychiatric side effects could reduce the motivation for neurorehabilitation and thus impair recovery after the stroke. Interactions with other drugs, such as anticoagulants, antihypertensives or cholesterol-lowering drugs, could reduce the efficacy of therapy and thus increase the risk of recurrent cerebrovascular events, especially with enzyme-inducing ASMs. Whether there is a relevant interaction between direct oral anticoagulants and ASMs is currently still unclear, as contradictory study results are available. Long-term treatment with ASMs may also increase the stigma and costs for patients.
Whether seizure suppressive treatment of acute symptomatic seizures after a stroke could have a neuroprotective or antiepileptogenic effect is still being investigated. There is no clear evidence to date.
In our view, an individual assessment is recommended. We follow the concept of “The Good, The Bad, and The Ugly”.
“The Good”: Acute symptomatic seizures with a good prognosis and a low risk are those that are not accompanied by severe impaired consciousness or pronounced motor phenomena and occur in patients who are only slightly impaired after the stroke and do not have high-grade vascular stenosis. If these seizures occur only once or rarely, we believe that seizure suppressive treatment is not necessary.
“The Bad”: If acute symptomatic seizures are associated with a risk of injury or neurological deterioration, e.g. high-grade stenosis, vasospasm or increased intracranial pressure, short-term seizure suppressive treatment should be considered. In these cases, it is advisable to use ASMs that can be dosed quickly and have a favorable interaction and side effect profile. Levetiracetam is most commonly used in practice (in >90% of cases) [21]. The second most common choice is lacosamide. Valproic acid is also used relatively frequently, but in our view it has a somewhat more problematic profile and should rather be regarded as a second-choice therapy.
In these cases, we recommend that treatment is only given for a short time, ideally for 7 to 14 days. In practice, however, such treatment is continued in some centers for 3 months, after which a decision is made to discontinue therapy in an outpatient consultation, including EEG.
“The Ugly”: In acutely symptomatic status epilepticus, there is a high risk (>80%) of subsequent epilepsy, poor outcome and high mortality [18]. In these cases, seizure suppressive treatment is always necessary. Due to the high risk of developing epilepsy later in life, long-term seizure suppressive treatment may also be considered. At the very least, regular neurological checks should be planned.
In addition to acute symptomatic status epilepticus, there are other situations with a high risk (>60%) of subsequent post-stroke epilepsy. These include tonic-clonic acute symptomatic seizures on the same day as the stroke as well as certain high-risk situations, such as the occurrence of epilepsy-typical changes in the early EEG. Common prognostic models such as SeLECT or CAVE should be used for precise risk stratification. If these models predict a risk of more than 60% for later epilepsy, some experts recommend long-term seizure suppressive treatment as if epilepsy were already present. However, whether this strategy is actually effective has not yet been investigated.
Prophylaxis of post-stroke epilepsy (antiepileptogenesis)
Epilepsy as a long-term consequence of cerebrovascular events is one of the most common causes of epilepsy in the elderly [9]. Although the risk of post-stroke epilepsy (PSE) within the first 10 years after an ischemic stroke is approximately 12% and even increases to 30% after a cerebral hemorrhage, these figures do not justify seizure suppressive therapy as prophylaxis without the actual occurrence of epileptic seizures [10,11]. Without corresponding evidence, such prophylactic therapy cannot be recommended because patients will suffer disadvantages due to side effects and no meaningful seizure protection. This problem is also exacerbated by the frequent polytherapy due to numerous comorbidities in this age group. Therefore, studies are needed that investigate pharmacological prophylaxis of the occurrence of PSE. And it is precisely such studies that are lacking today. The trials conducted to date have been rare and unsuccessful. In the first decade of this century, colleagues from Tel Aviv came up with a compact and promising design for the prevention of post-stroke epilepsy [12]. They chose patients with intracerebral hemorrhages as their target group, i.e. those with the highest risk of developing epilepsy. And the very potent seizure suppressant valproate was used as the pharmacological substance. A perfect constellation from the first glance to reach the goal. The authors randomized 72 patients with non-aneurysmal intracerebral hemorrhages into two groups in a double-blind fashion. One half (n=36) received valproate for 4 weeks and the other (n=36) placebo. The number of epileptic seizures within the first year after the cerebral hemorrhage was compared between verum and placebo and there was no statistically significant difference between the two arms (19.5% vs 22.2%, p=0.8).
Another study dealing with the question of antiepileptogenesis after stroke was “Early Treatment with Levetiracetam After Stroke for the prevention of late seizures” (ETLAS). A multicenter, randomized, placebo-controlled, double-blind study was planned in which stroke patients with a cortical syndrome and a modified Rankin score ≥3 or NIHSS ≥6 participated. Participants were treated with levetiracetam 1500 mg/day or placebo 12 weeks after stroke and followed up for 1 year [13]. Treatment was started between 48 hours and 7 days after the index event, and the primary endpoint was the occurrence of a first late epileptic seizure, defined as an unprovoked epileptic seizure more than one week after the stroke. Recruitment problems resulted in only 16 patients (levetiracetam, n = 9; placebo; n=7) being included in the study between August 2005 and December 2006 and only one patient (placebo arm) developing post-stroke epilepsy. Recruitment was made difficult because most patients met exclusion criteria or had comorbidities that prevented study participation. Due to these difficulties, the authors came to the conclusion that it is not possible to conduct an antiepileptogenesis study for the prevention of PSE.
Despite the unsuccessful attempts of the past decade, the prevention of post-stroke epileptogenesis remains a scientific challenge motivating new studies. Experimental animal models indicated a potential antiepileptogenic effect of eslicarbazepine acetate (ESL). This effect was hypothesized to be due to effective inhibition of high- and low-affinity hCaV3.2 inward currents [14,15]. For example, in a pilocarpine mouse model of chronic epilepsy, transient ESL treatment was shown to significantly reduce the frequency and duration of epileptiform discharges in the chronic stage, and it was additionally shown that ESL treatment attenuated neuronal loss and significantly reduced coordination impairment [14]. In this context, Prof. Koepp from London together with Prof. Trinka from Salzburg and other co-authors have designed a multicentre, randomized, double-blind, placebo-controlled phase II study to test the hypothesis of a possible preventive effect of ESL for the development of PSE and to assess whether a one-month ESL treatment can prevent unprovoked seizures after stroke [16]. Patients at high risk of developing unprovoked seizures after acute intracerebral hemorrhage or acute ischemic stroke were randomized to receive either ESL 800 mg/d or placebo, with treatment initiated within 120 hours of the onset of the primary stroke. Treatment was continued until day 30 and then tapered off. Patients were able to receive all necessary stroke treatment therapies according to clinical practice guidelines and standard of care and were followed up for 18 months. The primary endpoint was the occurrence of a first unprovoked seizure within 6 months of randomization (“failure rate”). Secondary endpoints include the occurrence of a first unprovoked seizure within 12 months of randomization and throughout the study period; functional outcomes (Barthel Index; NIHSS); post-stroke depression (Patient Health Questionnaire-9; PHQ-9); and overall survival. Safety assessments included evaluation of treatment-related adverse events; laboratory parameters; vital signs; electrocardiogram; suicidal ideation; and suicidal behavior (PHQ-9, question 9). The protocol aimed to randomize approximately 200 patients (1:1) recruited at 21 sites in seven European countries and Israel. Despite the challenges, especially during the COVID-19 pandemic, the study progressed and enrolled a remarkable number of patients: 129 were screened and 125 were randomized. Recruitment was stopped after 30 months and results are expected shortly.
Therapy for post-stroke epilepsy
The clinical course of PSE is heterogeneous. In addition to forms that respond well to therapy, there are also cases that are refractory to therapy. Specialist neurological care is therefore advisable for this disease. The experience of a specialist is required for the differential diagnosis of focal epileptic seizures from cerebrovascular events. Patients, but also doctors from other specialties, often confuse newly occurring ictal phenomena with transient ischemic attacks, which can lead to wrong therapeutic decisions. For this reason, neurological follow-up of patients who have had a stroke is recommended, as this allows secondary diseases to be correctly classified and treated.
Once PSE has been diagnosed, it is important to choose the right therapy to effectively control seizures and avoid side effects. As there are no specific guidelines for PSE, the choice of medication is generally based on recommendations for seizure suppressive therapy for structural (focal) epilepsies. However, this should take into account the comorbidity of stroke patients, as they often suffer from affective and cognitive disorders (e.g. post-stroke depression, vascular dementia). The administration of SV2A modulators, for example, can therefore lead to an increased risk of affective side effects. Overall, initial discontinuation of seizure suppressants requires specialist neurological monitoring and correction if necessary. Our studies have shown that the control and optimization of seizure suppressants in the first year after the onset of PSE leads to a 40% improvement in seizure severity and significantly increases the health-related quality of life of patients [17].
Data on the specific therapy of PSE is important to help patients quickly and effectively by choosing the right medication. While randomized double-blind studies on the treatment of PSE are lacking, several observational studies have addressed this issue. If not in prophylaxis, then in the treatment of diagnosed PSE, ESL is a promising substance. This is supported, for example, by the data from the Euro-Esli study. This is a pooled data analysis of 14 European studies, which included a total of 2058 patients [18]. In the current subgroup analysis, information on aetiology was known for 1656 patients and 76 (4.6%) of these had PSE. Seizure freedom and responder rate (≥50% reduction in seizure frequency) were taken as outcome parameters. At the last visit, the responder rate was significantly higher in patients with post-stroke epilepsy than patients with other etiologies of epilepsy (72.9% vs. 60.6%, p=0.04). There was no significant difference at other time points (3, 6 or 12 months). Seizure freedom was higher in favor of PSE at most time points (e.g. 48.6% vs. 31.7% at the last visit, p<0.01). The incidence of adverse events was similar in PSE patients compared to patients with other epilepsies (36.0% vs. 35.8%; p=0.966). The authors summarized that ESL could be an effective and well-tolerated treatment option for patients with focal seizures in PSE.
Our working group has endeavored to present a study in a clean monotherapy comparative design. A total of 207 patients were included, which is a large number of patients compared to other available studies [19]. The comparators were levetiracetam (n=60), lacosamide (n=43), lamotrigine (n=40), eslicarbazepine (n=38), valproate (n=18), topiramate (n=3), zonisamide (n=2), gabapentin (n=2) and carbamazepine n=1). The results showed that ASM with the mechanism of action via slow inhibition of sodium channels (eslicarbazepine and lacosamide) showed the best retention rate and seizure control compared to ASM with other mechanisms of action. As a self-criticism, it must be noted that the latest seizure suppressants, such as brivaracetam, perampanel and cenobamate, were not included in this study because treatment with these substances was not yet widespread at the time of data collection. The subgroup analysis of the BRIVAFIRST study with 75 PSE patients is now available for brivaracetam, which shows a 36-42.7% responder rate and 24-34.7% seizure freedom [20]. A comparison with other seizure suppressants was not carried out in this study.
Data on the treatment of status epilepticus in PSE are now available that replicate the results of our study cited above [19,21,22]. In 138 PSE patients with refractory status epilepticus, it was shown that seizure suppressants acting via slow inhibition of sodium channels were most effective in controlling status epilepticus [22]. In support of this, our study of the use of ESL to interrupt status epilepticus showed that the administration of these slow inhibitors of sodium channels was most effective in patients with PSE. Status was abolished by ESL application in 65.2% of patients with PSE compared to 29.8% in other forms of structural epilepsy (29.8%, p<0.01) [21].
Summary
Post-stroke epilepsy is the most common form of epilepsy in the elderly and can present both differential diagnostic and therapeutic challenges. For this reason, it is important to know the clinical presentation of epileptic seizures in the elderly and their mimics. Acute symptomatic seizures following a cerebrovascular event are associated with a low risk of recurrence and, unlike seizures as the first manifestation of epilepsy, are not usually an indication for long-term seizure suppressive therapy. The time limit of 7 days is used as a guideline, although exceptions are possible in special situations and a precise prediction of the probability of recurrence is the subject of current research. As a result of international initiatives, the SeLECT model, including its version available as a smartphone app for bedside use, has been developed for ischemic cerebral infarctions and the CAVE model for hemorrhagic infarctions. Based on calculated recurrence risk, decisions are made for the duration of seizure suppressive therapy and the possible scenarios (“The Good”, “The Bad” and “The Ugly”) were described in detail in this article. With regard to the prevention of epileptogenesis after a stroke, no positive research results are available to date, although this topic is still being investigated by various research groups. At present, the greatest hope lies in the slow inhibitors of sodium channels, such as eslicarbazepine or lacosamide. In terms of seizure suppressive effects, this mechanism of action certainly shows advantages in post-stroke epilepsy, as the latest studies with an open design show.
Take-Home-Messages
- Acute symptomatic post-stroke seizures generally do not require long-term therapy and the current recommended time limit in guidelines to differentiate epilepsy after stroke is 7 days.
- Validated instruments, such as the SeLECT model, have been developed for more precise assessment of the risk of recurrence of epileptic seizures after a stroke and are available digitally for use at the patient’s bedside.
- There is currently no evidence on the prevention of epileptogenesis after a stroke, although intensive research is currently being carried out on this topic.
- New studies with an open design provide initial indications that the mechanism of action of slow inhibition of sodium channels may have advantages over other seizure suppressants in terms of seizure suppression in post-stroke epilepsy.
Literature:
- Misra S, Kasner SE, Dawson J, et al: Outcomes in Patients With Poststroke Seizures. JAMA Neurol 2023; 80(11): 1155-1165. doi:10.1001/jamaneurol.2023.3240.
- Beghi E, Carpio A, Forsgren L, et al: Recommendation for a definition of acute symptomatic seizure. Epilepsia 2010; 51(4): 671-675. doi:10.1111/j.1528-1167.2009.02285.x.
- Fisher RS, Acevedo C, Arzimanoglou A, et al. ILAE official report: a practical clinical definition of epilepsy. Epilepsia 2014; 55(4): 475-482. doi:10.1111/epi.12550.
- Holtkamp M, May TW, Berkenfeld R, et al. S2k guideline: First epileptic seizure and epilepsies in adulthood. DGNeurology. 2024;7(1):21-38. doi:10.1007/s42451-023-00618-z
- Peter-Derex L, Philippeau F, Garnier P, et al: Safety and efficacy of prophylactic levetiracetam for prevention of epileptic seizures in the acute phase of intracerebral haemorrhage (PEACH): a randomized, double-blind, placebo-controlled, phase 3 trial. Lancet Neurology 2022; 21(9):781-791. doi:10.1016/s1474-4422(22)00235-6.
- Galovic M, Döhler N, Erdélyi-Canavese B, et al: Prediction of late seizures after ischaemic stroke with a novel prognostic model (the SeLECT score): a multivariable prediction model development and validation study. Lancet Neurol 2018; 17(2): 143-152. doi:10.1016/s1474-4422(17)30404-0.
- Sinka L, Abraira L, Imbach LL, et al: Association of Mortality and Risk of Epilepsy With Type of Acute Symptomatic Seizure After Ischemic Stroke and an Updated Prognostic Model. JAMA Neurol 2023; 80(6): 605-613. doi:10.1001/jamaneurol.2023.0611.
- Haapaniemi E, Strbian D, Rossi C, et al: The CAVE score for predicting late seizures after intracerebral hemorrhage. Stroke. 2014;45(7):1971–1976. doi:10.1161/strokeaha.114.004686
- Cloyd J, Hauser W, Towne A, et al: Epidemiological and medical aspects of epilepsy in the elderly. Epilepsy Res 2006 Jan; 68 Suppl 1: S39-48.
- Graham NS, Crichton S, Koutroumanidis M, et al: Incidence and associations of poststroke epilepsy: the prospective South London Stroke Register. Stroke 2013 Mar; 44(3): 605-611.
- Arntz R, Rutten-Jacobs L, Maaijwee N, et al: Post-stroke epilepsy in young adults: a long-term follow-up study. PLoS One 2013; 8(2): e55498.
- Gilad R, Boaz M, Dabby R, et al: Are post intracerebral hemorrhage seizures prevented by anti-epileptic treatment? Epilepsy Res 2011 Aug; 95(3): 227-231.
- van Tuijl JH, van Raak EP, de Krom MC, et al: Early treatment after stroke for the prevention of late epileptic seizures: a report on the problems performing a randomized placebo-controlled double-blind trial aimed at anti-epileptogenesis. Seizure 2011; 20: 285-291.
- Doeser A, Dickhof G, Reitze M, et al: Targeting pharmacoresistant epilepsy and epileptogenesis with a dual-purpose antiepileptic drug. Brain 2015; 138: 371-387.
- Soares-da-Silva P, Pires N, Bonifacio MJ, et al: Eslicarbazepine acetate for the treatment of focal epilepsy: an update on its proposed mechanisms of action. Pharmacol Res Perspect 2015; 3: e00124.
- Koepp MJ, Trinka E, Mah YH, et al: Antiepileptogenesis after stroke-trials and tribulations: Methodological challenges and recruitment results of a Phase II study with eslicarbazepine acetate. Epilepsia Open 2023 Sep; 8(3): 1190-1201.
- Winter Y, Daneshkhah N, Galland N, et al: Health-related quality of life in patients with poststroke epilepsy. Epilepsy & Behavior 2018; 80: 303-306
- Villanueva V, Holtkamp M, Delanty N, et al: Euro-Esli: a European audit of real-world use of eslicarbazepine acetate as a treatment for partial-onset seizures. J Neurol 2017; 264: 2232-2248.
- Winter Y, Uphaus T, Sandner K, et al: Efficacy and safety of antiseizure medication in post-stroke epilepsy. Seizure. 2022 Aug; 100: 109-114.
- Lattanzi S, Canafoglia L, Canevini MP, et al: BRIVAFIRST Group Membership. Brivaracetam as add-on treatment in patients with post-stroke epilepsy: real-world data from the BRIVAracetam add-on First Italian netwoRk Study (BRIVAFIRST). Seizure. 2022 Apr; 97: 37-42.
- Winter Y, Sandner K, Vieth T, Groppa S: Eslicarbazepine acetate for the treatment of status epilepticus. Epileptic Disord 2023 Apr; 25(2): 142-149.
- Winter Y, Sandner K, Vieth T, et al: Third-Generation Antiseizure Medication in the Treatment of Benzodiazepine-Refractory Status Epilepticus in Poststroke Epilepsy: A Retrospective Observational Register-Based Study. CNS Drugs 2023 Oct;37(10): 929-936.
InFo NEUROLOGY & PSYCHIATRY 2024: 22(6): 8-13