Clinical symptoms are critical in assessing the course and therapy of multiple sclerosis. To date, no symptom assessment method has been able to replace the clinical neurological examination, and no clinical measurement tool can better quantify the totality of deficits than the neurostatus (EDSS). Neurostatus e-scoring, which was introduced in 2011 and which has an instant feedback function, could lead to a relevant improvement in reliability. Neurostatus should be used regularly in routine clinical practice so that examination results in different patients can be compared across the boundaries of one’s own hospital.
Clinical assessment of limitation by symptoms of multiple sclerosis (MS) forms the basis for defining the course of the disease (relapsing or progressive) and selecting therapy – including non-drug procedures. In the 1970s, the World Health Organization (WHO) classified the limitations that result from a disease in humans and introduced the terms impairment, disability and handicap. This WHO classification is also mostly the basis for the clinical measurement instruments used in MS, but the objective recording of limitations is only partially possible.
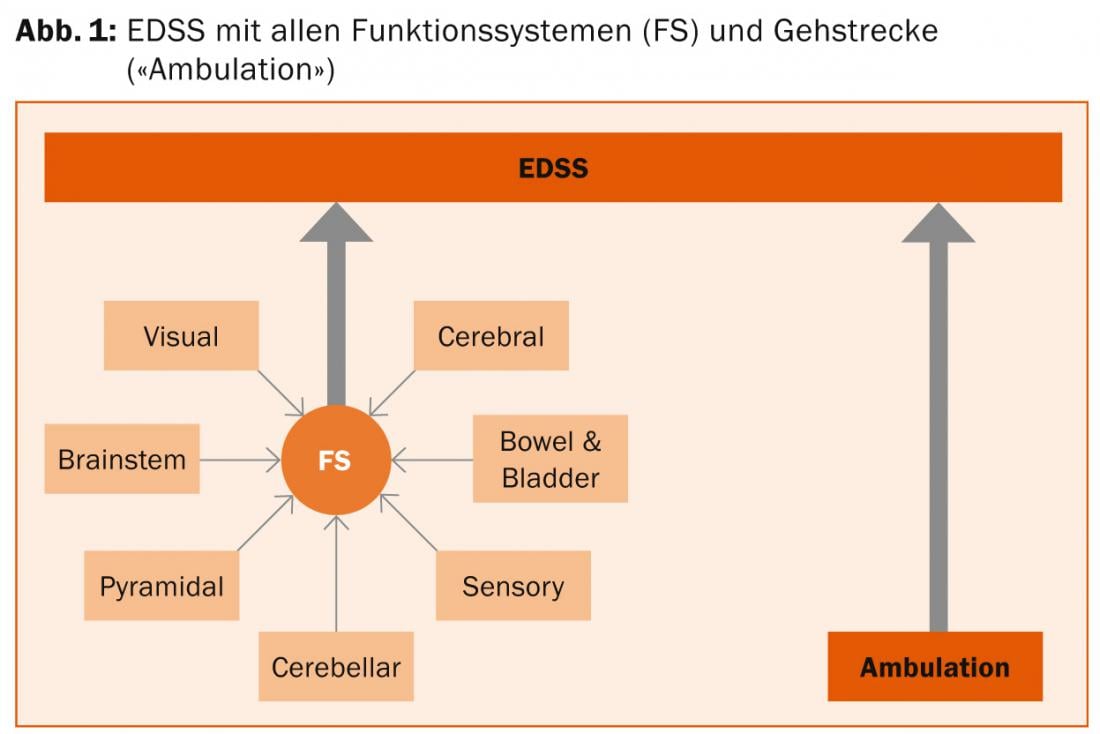
Development of the Expanded Disability Status Scale (EDSS).
The Expanded Disability Status Scale (EDSS) focuses on clinical neurological assessment of symptoms. As the most widely used of the clinical scales, the EDSS can still best meet the WHO specifications. Neither laboratory chemical analyses of blood and CSF nor imaging (MRI) or evoked potentials derivation can sufficiently reliably depict the clinical course of the disease over time [1–3]. The use of “patient reported outcomes” (PRO) is too prone to interference to be used as an objectifiable outcome parameter, for example in clinical trials or for the evaluation of a drug change [4].
Historically, the development of the EDSS begins as the DSS (“Disability Status Scale”) in the 1950s. Dr. John Kurtzke, an American neurologist, sought a clinical evaluation procedure to test the potential therapeutic success of isoniazid in MS therapy as part of a clinical trial [5]. The measurement instruments available until then were not suitable for such a study. Functional systems (FS) were added to the DSS to quantify symptoms [6]. However, the study was not very successful for isoniazid. In the following years, modifications were made to the FS by Kurtzke, and to increase sensitivity, he expanded the original eleven degrees of the DSS (0-10) by half-steps to a total of 20 (0; 1; 1.5; 2, etc. to 10) [7].
Acquisition of seven functional systems
The seven functional systems, in turn, consist of various subsystems and are recorded in the course of a clinical-neurological examination:
- Visual symptoms (Visual FS), including by means of a high-contrast vision chart and finger perimetry
- Cranial Nerve Failures (Brainstem FS)
- Paresis and spasticity (Pyramidal FS)
- Coordination disorders (Cerebellar FS).
- Decrease in perception of touch and proprioception (Sensory FS).
- Disorders of the urinary bladder, rectum, and genital organs (Bowel and Bladder FS).
- Change in mood, cognition, and incidence of fatigue (Cerebral FS).
The EDSS value is defined by the combination of different FS values (regardless of which system the FS value originates from) plus the patient-reported walking distance (Fig. 1). In particular, a walking distance of less than 500 m has a clear influence on the EDSS. In the lower values, the EDSS predominantly captures Impairment and in the higher values, Disability. Only little is recorded the handicap.
Improvement of reliability and consistency
Although the core of the EDSS is the neurological examination, objectively recordable findings (e.g., ocular motor function) are mixed with purely subjective reportable findings (e.g., fatigue). In Kurtzke’s version, the walking distance only reported and the definitions of FS and their subsystems, which were not used with much precision, were found to be disadvantages. The determination of EDSS values >4.0 from FS values and walking distance was also ambiguous. For these reasons, the EDSS lacked good reliability between different examiners (“low inter-rater reliability”) and with one examiner over time (“low intra-rater reliability”) [1–3].
In order to increase the reliability and consistency of the quantitative assessment of symptoms, the “neurostatus” was developed in the 1990s. Based on the EDSS and a consensus of the leading MS specialists from Europe and North America at that time, the neurological examination was standardized; in addition, clearer definitions for the graduation and determination of FS and its subsystems were introduced (Fig. 2). In addition, conversion tables were introduced for the “Visual FS” and the “Bowel and Bladder FS” so that different impairments in quality of life could be compensated with the same FS values in the case of significant functional impairment compared with the other FS. In addition, the walking distance up to 500 m must be tested as part of the examination. EDSS 6.0 and 6.5 were better differentiated by type of walking aid (unilateral or bilateral) as well as length of walking distance possible with it during the examination and replaced the original definition of EDSS. In subsequent years, the definitions of this standardized EDSS, neurostatus, have been refined several times, most recently in 2011 [8].

Standardized EDSS trainings conducted by experienced neurologists of the University Hospital Basel at clinical trial events as well as an interactive training DVD-ROM, including a website with information about neurostatus (www.neurostatus.net, including a question and answer forum) lead to a further increase in the consistency of the surveyed
Data.
Regular “quality control” of “trained” EDSS raters (“raters”) has occurred since the introduction of Neurostatus e-Test certification in 2003. Here, 25 questions have to be answered in an online test, which exclusively concern the calculation of FS and EDSS values. Depending on the number of correctly answered questions, a certificate for level A, B or (highest) C is awarded. A standardized EDSS assessment by telephone was also added [9].
Electronic recording of the EDSS
Neurostatus has been used in more than 130 Phase II and III clinical trials over the past two decades, including most of the trials that led to the approval of current MS drugs. Since the introduction of Neurostatus, more than 9000 EDSS raters have been certified. To further improve reliability, Neurostatus e-Scoring (NESC), the electronic EDSS, was introduced in 2011. Based on the neurostatus definitions from 2011, an algorithm was programmed in close collaboration with Neurostatus Systems GmbH and employees of the MS Center in Basel, which enables the precise determination of the FS values from the neurostatus subsystems as well as the EDSS value from given FS values and the walking distance (Fig.3). The NESC is already being used successfully in three clinical trials.
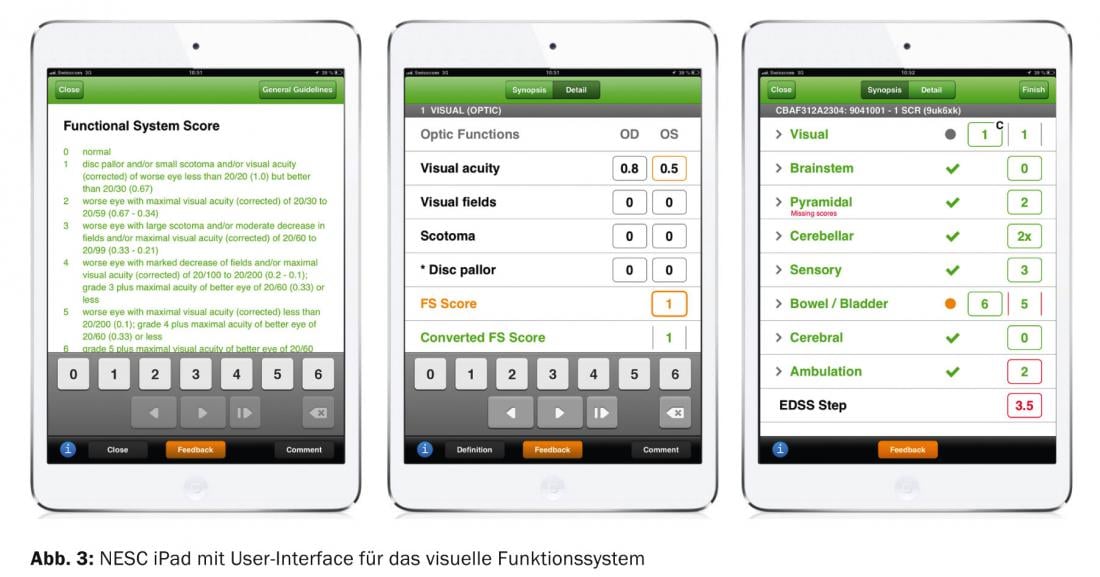
In addition, when implemented on an iPAD, the NESC provides an instant feedback function of the calculation of FS and EDSS values made by the EDSS rater. In addition, cross-checks exist to determine whether examination findings are consistent or inconsistent; for example, inconsistent would be a report of high-grade paralysis of the legs with a normal walking distance.
Use in studies and in practice
Increasingly, clinical course parameters, mostly EDSS scores, are used as primary endpoints in drug trials. As an indicator of sustained progression, the following value is commonly used in clinical trials: increase in EDSS value by ≥1 point from baseline measurement, sustained over three or six months. For diagnosis and evaluation of the right therapy, the qualitative and especially quantitative determination of MS-related symptoms is essential. No procedure to date can replace the clinical neurological examination, and no clinical measurement tool to date can better quantitatively assess the totality of the deficits that occur than the EDSS/neurostatus. Despite various attempts to develop alternative clinical measurement tools [1,2], it has not yet been possible to replace the EDSS. The EDSS is the only scale accepted by both the U.S. FDA and the European Medicines Agency (EMA) [1].
However, neurostatus should not only be used in clinical trials, but also as part of daily routine so that examination results can be compared between different patients and neurologists across the boundaries of one’s own hospital. Neurostatus is also used in multicenter observational studies, such as MSBase (www.msbase.org) and in Switzerland in the Swiss Multiple Sclerosis Cohort Study (SMSC). Thus, more experience can be gained regarding the effects and side effects of MS therapeutic procedures.
Marcus D’Souza, MD
Athina Papadopoulou, M.D.
Prof. Dr. med. Ludwig Kappos
Literature:
- Cohen J, et al: Disability outcome measures in multiple sclerosis clinical trials: current status and future prospects. Lancet Neurol 2012; 11: 467-476.
- Sharrack B, Hughes R: Clinical scales for multiple sclerosis. Journal of the Neurological Sciences 1996; 135: 1-9.
- D’Souza M, Kappos L, Czaplinski A: Reconsidering clinical outcomes in multiple sclerosis: relapses, impairment, disability and beyond. Journal of the Neurological Sciences 2008; 274: 76-79.
- Mayo N, Hum S, Kuspinar A: Methods and measures: what’s new for MS? Mult Scler 2012; 19(6): 709-713.
- Kurtzke JF: A new scale for evaluating disability in sclerosis. Neurology 1955; 5: 580-583.
- Kurtzke JF: On the evaluation of disability in multiple sclerosis. Neurology 1991; 11: 686-694.
- Kurtzke JF: Rating neurologic impairment in multiple sclerosis: An expanded disability status scale (EDSS). Neurology 1983; 33: 1444-1452.
- Kappos L: Neurostatus Scoring Version 04/10.2. 2011.
- Lechner-Scott J, et al: Can the Expanded Disability Status Scale be assessed by telephone? Mult Scler 2003; 9(2): 154-159.
InFo NEUROLOGY & PSYCHIATRY 2014; 12(6): 6-9.