Children with type 1 diabetes have an increased risk of developing diabetic kidney disease (DKD). Inhibition of dipeptidyl peptidase-4 (DPP4) has positive effects on various metabolic indicators in diabetes. Egyptian researchers investigated the effect of sitagliptin on diabetic nephropathy in adolescents with type 1 diabetes and nephropathy when used as an add-on therapy to the advanced hybrid closed-loop (AHCL) system.
The incretin-based (GLP1 receptor agonists and DPP4 inhibitors such as sitagliptin are used as blood glucose-lowering therapies in type 2 diabetes mellitus. Numerous clinical studies have demonstrated the beneficial therapeutic effects of DPP4 inhibitors in type 1 diabetes, where they lowered prandial insulin dose and total daily insulin dose (TDD), inhibited glucagon secretion and lowered blood glucose levels. In addition, DPP4 inhibitors exert renoprotective effects in diabetic nephropathy through glucose-dependent and glucose-independent mechanisms.
Dr. Nancy S. Elbarbary from the Department of Pediatrics, Faculty of Medicine, Ain Shams University, Cairo, and colleagues conducted a randomized controlled trial evaluating the role of sitagliptin as adjunctive therapy for early diabetic nephropathy in adolescents with type 1 diabetes undergoing AHCL [1]. They also evaluated the association with microalbuminuria, stromal cell-derived factor-1 ( SDF-1 ), lipid profile and AHCL glucometry. It is the first study to investigate the role of sitagliptin in adolescents with diabetic nephropathy under an AHCL regimen.
46 participants with type 1 diabetes and diabetic nephropathy aged 11 to 18 years who had been treated with the MiniMed 780G system for at least 6 months prior to the study and had an HbA1c level of ≤69 mmol/mol (8.5%) were included. None of the participants were hypertensive or obese. They were randomly assigned to two groups (n=23 each) based on a computer-generated randomization sequence. The intervention group received 50 mg sitagliptin orally for 3 months. The control group was on AHCL only. The primary endpoint was the change in urinary albumin/creatinine ratio (UACR) after 3 months of sitagliptin administration. The main secondary endpoint was the change in SDF-1 levels after treatment compared to baseline.
In addition to pharmacological treatment, nutrient intake was tabulated using a 24-hour dietary reminder list, which was carried out directly by a dietician by interviewing the adolescents or their caregivers. All participants were advised to eat a regular, balanced diet with an optimal macronutrient distribution.
All participants were asked to calculate the amount of carbohydrate in their meals and to take a pre-bolus beforehand. They were also followed up clinically every 4 weeks for 3 months during the study period to monitor possible adverse effects such as gastrointestinal symptoms, upper respiratory tract infections and skin reactions. Metabolic events such as hypoglycemia or diabetic ketoacidosis (DKA) were recorded during the study. At the end of the three-month period, participants were examined and UACR and SDF-1 levels were measured.
DPP4 inhibitor improves blood glucose control and reduces UACR
When comparing the clinical and laboratory data at baseline, there were no significant differences between the two groups, not even with regard to the settings of the MiniMed 780G AHCL system, ease of use or glucometry (p>0.05).
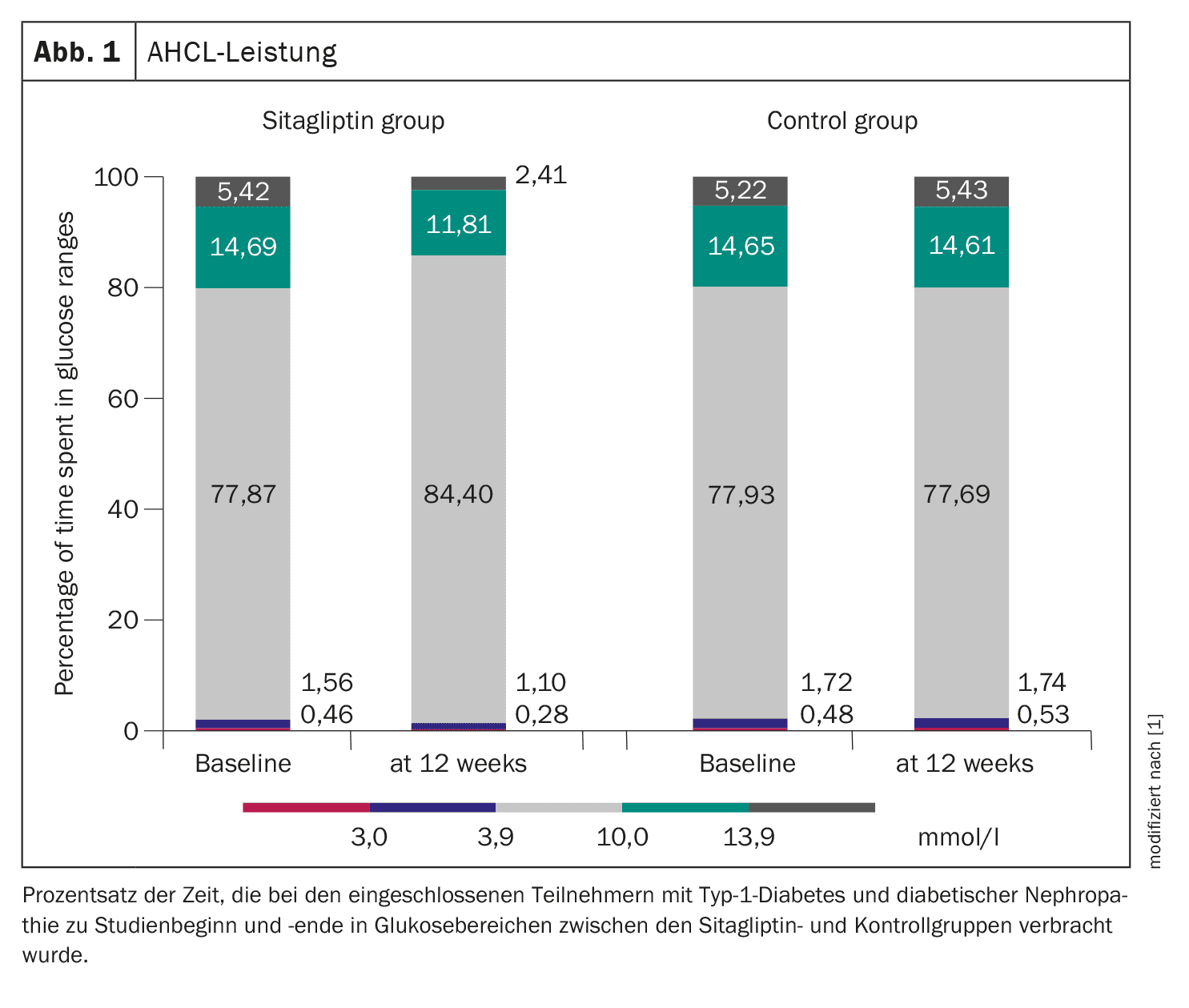
Serum SDF-1 levels from all subjects were compared to a healthy cohort with type 1 diabetes and were found to be elevated (p<0.001). After 3 months, sitagliptin resulted in a significant decrease in SDF-1 levels from 3.58 ± 0.73 to 1.99 ± 0.76 ng/mL (p<0.001), along with an improvement in UACR from 7.27 ± 2.41 to 1.32 ± 0.31 mg/mmol (p<0.001). In addition, sitagliptin reduced postprandial glucose, sensor glucose, coefficient of variation and total daily insulin dose, while time in range (TiR) 3.9-10.0 mmol/l (70-180 mg/dl) and insulin/carbohydrate ratio (ICR) were significantly increased (TiR from 77.87 ± 4.23% to 84.40 ± 5.15%). The time below range (TbR) <3.9 mmol/l was reduced from 1.56 ± 0.41% to 1.10 ± 0.17% and TbR <3.0 mmol/l from 0.46 ± 0.21% to 0.28 ± 0.1%, while the time above range (TaR) 10.0-13.9 mmol/l was reduced from 14.69 ± 3.84% to 11.81 ± 2.87% and the TaR >13.9 mmol/l was reduced from 5.42 ± 1.33% to 2.41 ± 0.99% (Fig. 1). No subjects experienced severe hypoglycemia or diabetic ketoacidosis, five participants had skin irritations associated with the use of the sensor, which could be resolved locally with a cream.
SDF-1 is localized in podocytes and distal tubule cells of human kidneys and is secreted under the influence of hyperglycemia or ischemic renal injury. Although SDF-1 can alleviate renal damage, it promotes leukocyte infiltration and aggregation of inflammatory cells as well as the potentiation of chemokines that contribute to a proliferative response in the kidney. All of these factors ultimately lead to glomerular sclerosis, podocyte loss, albuminuria and DKD.
In the Egyptian researchers’ study, three months of add-on therapy with sitagliptin resulted in a significant reduction in serum SDF-1 levels and UACR, while eGFR improved after therapy compared to baseline values and the control group. According to Dr. Elbarbary and colleagues, these results suggest that sitagliptin may have a renoprotective effect in individuals with type 1 diabetes using the MiniMed 780G AHCL system.
It has been reported that the renoprotective effects of DPP4 inhibitors may be due to an increased half-life of their substrates such as GLP1 and SDF-1a. The researchers point out that their participants used an AHCL system and had relatively good glycemic control. Both UACR and SDF-1 were positively correlated with blood glucose, and the improvement in renal parameters could be related to improved glycemic outcomes; however, the possibility of a direct effect in these individuals could not be ruled out. The literature has shown that DPP4 inhibitors may improve two important risk factors for diabetic nephropathy – hyperglycemia and albuminuria. This means that beyond glycemic control, there are potentially positive effects on the kidney.
Reduction in carbohydrate intake
Both groups were followed up by Dr. Elbarbary and colleagues at the same intervals. However, further adjustments to the ICR in the intervention group (to be less tense) helped to minimize the number of hypoglycemic episodes and reduce the insulin dose. In addition, a reduction in carbohydrate intake/intake was observed between the sitagliptin and control groups in their study. A possible reason for this could be the pharmacological effects of GLP1, such as delayed gastric emptying, which promotes satiety and leads to a feeling of fullness after eating, which ultimately reduces carbohydrate intake.
Sitagliptin at a dose of 50 mg orally per day for 3 months as an add-on to the AHCL system for adolescents with type 1 diabetes and diabetic nephropathy improved blood glucose levels and TiR while lowering glycemic variability, insulin dose, UACR, and SDF-1, resulting in a renoprotective effect in these participants, Dr. Elbarbary and her colleagues concluded. However, further studies with longer follow-up periods of sitagliptin therapy are needed to verify the results and investigate their full efficacy and safety profiles, as well as the long-term effects on the progression of kidney disease and other diabetic complications, they said.
Literature:
- Elbarbary NS, et al.: The DPP-4 inhibitor sitagliptin improves glycaemic control and early-stage diabetic nephropathy in adolescents with type 1 diabetes using the MiniMed 780G advanced hybrid closed-loop system: a randomised controlled trial. Diabetologia 2024; doi: 10.1007/s00125-024-06265-7.
InFo DIABETOLOGIE & ENDOKRINOLOGIE 2024; 1(4): 30–32