The distinction between IgA nephropathy and lupus nephritis in patients with systemic lupus erythematosus (SLE) has important prognostic and therapeutic implications.
Physicians from China describe a case of systemic lupus erythematosus with IgA nephropathy and acute progressive glomerulonephritis and explained the relationship
between the two.
A 72-year-old man presented to the team of Dr. Zhifeng Jiang, Xiaogan Hospital, Wuhan University, China, due to loss of appetite and fatigue that had persisted for a week [1]. The patient had suffered from hypertension for years, but had no other chronic health problems and did not take medication regularly. There was no Raynaud’s phenomenon, no finger swelling, no rash, no joint pain, no cough and no wheezing. His blood pressure was 138/94 mmHg and he had bilateral edema in the lower extremities. The facial skin was not damaged, the fingers of both hands were not swollen and the joints were not deformed or painful. Laboratory tests revealed antinuclear antibodies (positive), leukocytes, red blood cells, thrombocytopenia, decreased complement C3, renal dysfunction and anti-SM positivity. The results of the renal ultrasound showed increased echogenicity of both renal parenchyma, the computerized tomography of the chest showed a small pleural effusion.
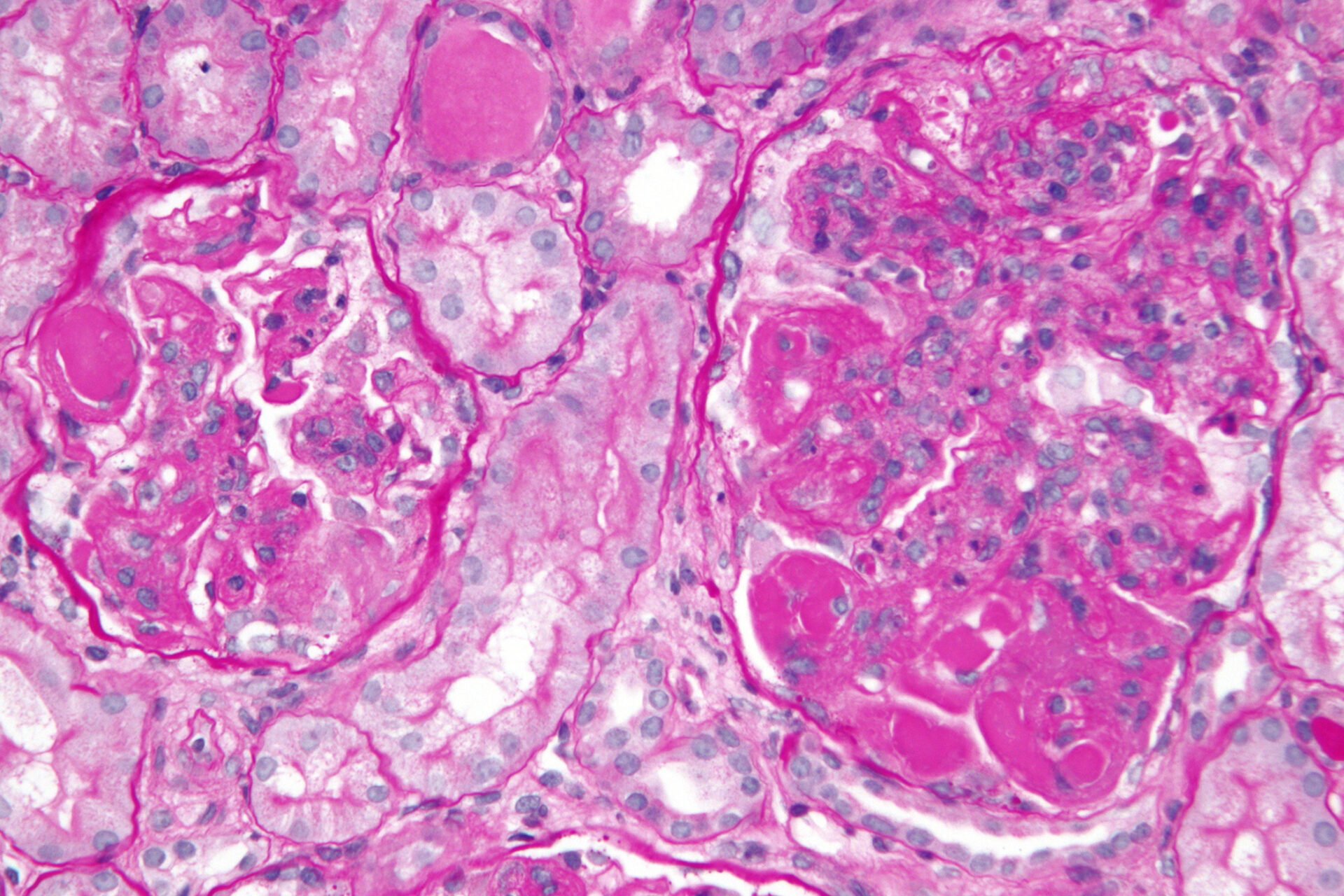
After admission, the patient’s renal function continued to deteriorate, and daily urine output was less than 50 ml/d. Two days later, the serum creatinine increased to 1108 μmol/l, and on the third day after admission, a temporary dialysis catheter was placed in the right internal jugular vein and hemodialysis was performed. The results of a renal biopsy (after three times of dialysis) showed a large number of cellular crescents, and although a large amount of IgA deposition was found in the mesangial area under immunofluorescence, there were no electron-dense deposits under the epithelium, basement membrane, and endothelium. Electron microscopy revealed electron-dense deposits in the mesangial area (Fig. 1A-D).
SLE and IgA nephropathy can occur simultaneously
The doctors diagnosed SLE with IgA nephropathy and acute glomerulonephritis.
On the tenth day after admission, high-dose glucocorticoid pulse therapy (methylprednisolone sodium succinate 500 mg for 3 days) and cyclophosphamide pulses (600 mg once daily for 2 days) were administered.
Subsequently, methylprednisolone sodium succinate 60 mg was administered once daily.
Hydroxychloroquine or angiotensin-converting enzyme inhibitors were not used.
After about one week, a routine blood test showed that platelets were 140 × 109/l, hemoglobin and complement C3 were 0.14 g/l.
At the 3-month follow-up, the patient still had anuria, renal function had not recovered, and he continued to be dialyzed.
Renal function did not further recover even after the administration of glucocorticoids and cyclophosphamide pulse therapy, demonstrating that systemic lupus erythematosus and IgA nephropathy can occur simultaneously.
SLE with IgA nephropathy and crescentic nephritis is rare, the relationship between the two conditions is unclear, and there are no clear treatment recommendations for these patients, the Chinese authors write.
There are several reports of histologically confirmed IgA nephropathy in patients with systemic lupus erythematosus and IgA nephropathy detected by renal biopsy in patients with inactive lupus.
Episodes of IgA nephropathy due to systemic inflammatory damage are a common feature in these patients.
Most affected individuals have normal complement levels, but in some cases reduced levels are seen, presumably due to extrarenal lupus activity.
Dr. Jiang and colleagues emphasize that in their patient after high-dose glucocorticoid and cyclophosphamide shock treatment, the increase in complement C3 concentration and platelet count supports the diagnosis of systemic lupus erythematosus, but the patient’s non-improving renal function supports the independence of IgA nephropathy and SLE; At the same time, the patient’s renal function deteriorated rapidly, and multiple cellular crescents formed under the light microscope, suggesting that the patient had an acute onset and progressed to end-stage renal failure.
There is currently no recommended treatment plan for IgA nephropathy associated with acute progressive glomerulonephritis. The lack of success after high-dose glucocorticoid and cyclophosphamide shock therapy in this case suggests that such patients have a poor prognosis on conventional glucocorticoid and immunosuppressive therapy. Sparsentan is a novel non-immunosuppressive single molecule; it has been reported that sparsentan can reduce proteinuria and delay the deterioration of renal function in patients with IgA nephropathy associated with sickle body formation. However, its use in IgA nephropathy with acute neoglomerulonephritis has not been reported; sparsentan may be applicable in such cases in the future, although further clinical studies are required.
When systemic lupus erythematosus and IgA nephropathy occur at the same time, there is no consensus as to whether IgA nephropathy and SLE occur independently of each other or not. The authors state that further cases and more biopsies may be able to shed light on this in the future. Furthermore, they conclude that systemic lupus erythematosus with kidney damage is not necessarily lupus nephritis and that the diagnosis of lupus nephritis must be confirmed by a biopsy.
Literature:
- Jiang Z, Feng A: A Case Report of Systemic Lupus Erythematosus With IgA Nephropathy and Crescentic Nephritis. AIM Clinical Cases 2023; 2: e230157; doi: 10.7326/aimcc.2023.0157.
InFo RHEUMATOLOGIE 2024; 6(1): 24-25
Cover picture: @Nephron, wikimedia