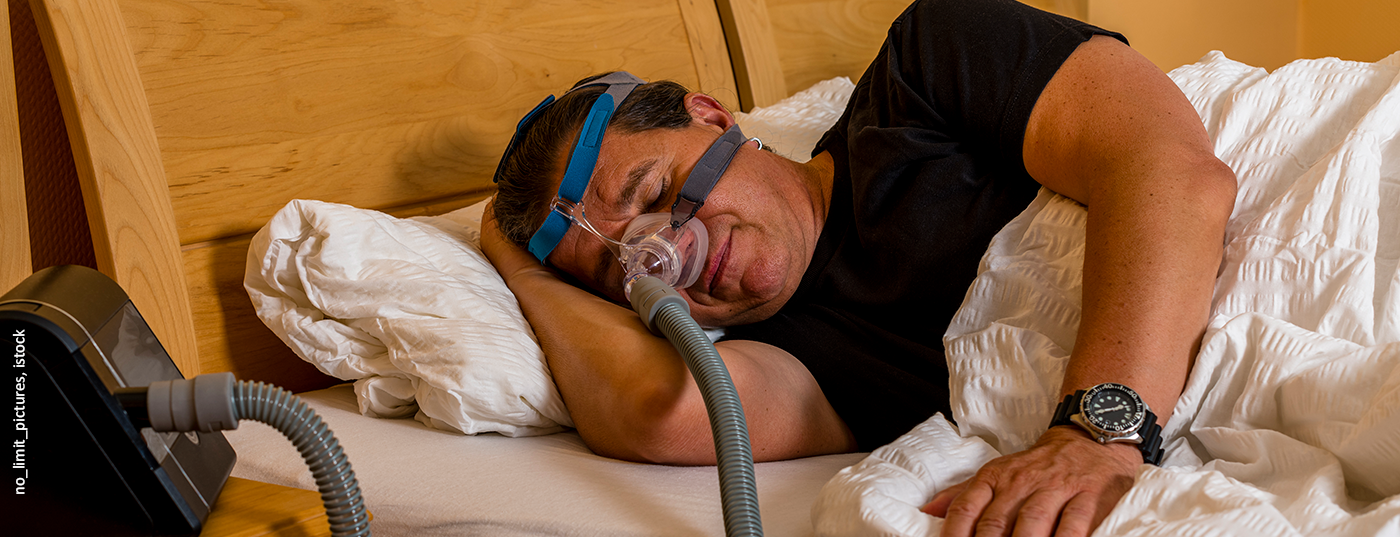
Sleep-related breathing disorders are common. Obstructive sleep apnea (OSA) has the character of a widespread disease. The prevalence of obstructive sleep apnea is steadily increasing in Western industrialized countries due to the increasing prevalence of obesity, aging, and the widespread use of diagnostic methods. 40% of the population suffers from obstructive sleep apnea with an apnea hypopnea index (AHI) >5, where AHI describes the occurrence of respiratory events per hour of sleep.
Sleep-related breathing disorders are common. Obstructive sleep apnea (OSA) has the character of a widespread disease. The prevalence of obstructive sleep apnea is steadily increasing in Western industrialized countries due to the increasing prevalence of obesity, aging, and the widespread use of diagnostic methods. 40% of the German population suffers from obstructive sleep apnea with an Apnea Hypopnea Index (AHI) >5, where AHI describes the occurrence of respiratory events per hour of sleep.
The disease is more common in men and older people over the age of 60. OSA syndrome, the combination of OSA and concomitant daytime sleepiness, is reported to have a prevalence of five percent of the German population (no current surveys are currently available from Switzerland) [1]. Untreated sleep-related breathing disorders lead to cardiovascular sequelae, pronounced daytime sleepiness or daytime drowsiness, and thus to an increased risk of accidents. The cognitive function of the affected person may also be affected.
According to the ICSD-3, sleep-disordered breathing is divided into four major groups: obstructive SA, central SA, sleep-related hypoxemia, sleep-related hypoventilation [2]. In OSA, repetitive upper airway collapse occurs. Due to the decreased muscle tone, it occurs more frequently in deep and REM sleep. If a collapse of the upper airway occurs in the form of apnea, the affected person tries to work against this apnea with increased respiratory effort. This increased effort is followed by a central nervous arousal response, called arousal. If the affected person succeeds in reopening the airways, a loud irregular snoring sound is produced. Central sleep apnea results from a disorder of central respiratory regulation. Sleep-related hypoxemia and hypoventilation result from a persistent decrease in respiratory activity. What they all have in common is the disturbance of the normal sleep architecture, i.e. sleep is constantly interrupted by wake-up reactions, the proportion of deep and REM sleep decreases, resulting in an unrestful sleep with a resulting daytime tiredness or sleepiness.
Symptoms and diagnostics
Breathing pauses, snoring, and daytime sleepiness are reported as common symptoms of sleep-disordered breathing; in this case, it is particularly necessary to obtain information from others, since it is not uncommon for affected patients to report no subjective complaints. Insomniac complaints are indicated by fragmentation of sleep architecture. In cases of pronounced apnea, an awakening with shortness of breath is described. Questionnaires can be used to assess daytime sleepiness resulting from SBAS (sleep disordered breathing). The Epworth Sleepiness Scale is often used for this purpose. Here, patients are asked about their likelihood of falling asleep in eight typical everyday situations. The individual results are summed to a total value between zero and 24. A value above ten is considered pathological. Clinical examination should include inspection of the upper airway. The nasal cavity, nasopharynx, oral cavity, and deep pharynx and larynx should be examined. In particular, anatomical obstruction in the oral cavity, especially hypertrophy of the palatine tonsils and the base of the tongue, play a special role in the development of sleep-disordered breathing in the sense of OSA.
The position of the upper jaw in relation to the lower jaw and the dental status should also be assessed. Both cardio-respi-ratory polygraphy and polysomnography are available as apparative diagnostics. As a basic diagnostic procedure, polygraphy is initially performed on an outpatient basis, i.e. the patient can sleep with the diagnostic system in his or her familiar environment at home. The PG device is a portable meter that measures or records nasal airflow, chest and abdominal respiratory excursions, heart rate, snoring, oxygen saturation, and patient position. The data is recorded continuously over a period of at least 6 hours and then processed or synchronized with software so that the attending physician can perform an individual evaluation of the entire night. If a diagnosis of sleep-disordered breathing is not possible during outpatient cardiorespiratory polygraphy, the affected patient must be referred to a sleep laboratory for further diagnosis, in this case polysomnography.
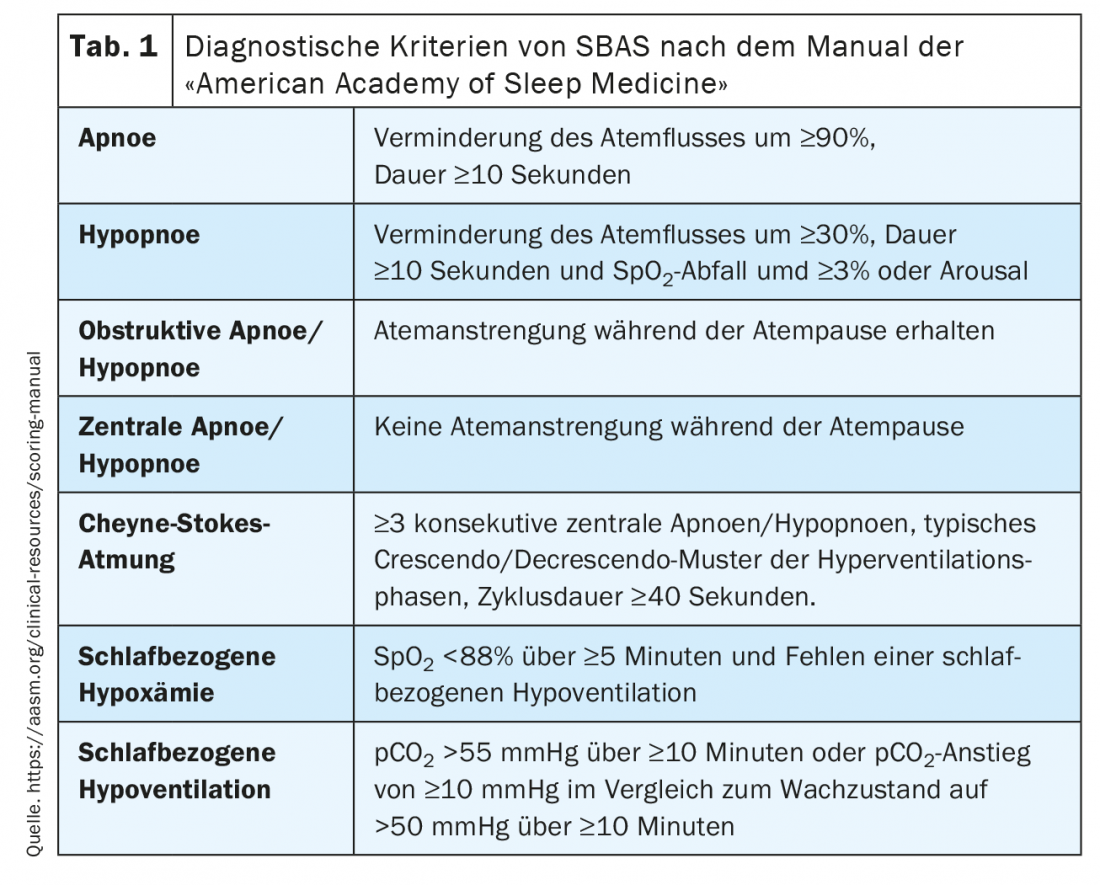
The diagnostic criteria of SBAS in the context of PG/PSG are defined according to the American Academy of Sleep Medicine manual [3] and are summarized in Table 1 .
Obstructive sleep apnea
OSA results from obstruction of the upper airway in the posterior (oro)pharyngeal region during sleep. The cause may be a pronounced so-called webbing of the soft palate with deep palatal arches and a possibly widened and elongated uvula, hypertrophy of the palatine tonsils or hypertrophy of the base of the tongue. Similarly, jaw malpositions or non-anatomical factors such as weakness of the genioglossus muscle, which has a dilating function on the pharyngeal portion of the upper airway, occur. Most commonly, airway narrowing occurs in association with obesity, with increased fat storage in the pharyngeal sidewalls. Obesity is the main risk factor of OSA along with increasing age and male gender. Accordingly, patients with metabolic syndrome are particularly frequently affected by OSA [4]. OSA is the most common cause of secondary arterial hypertension and affects diabetic metabolic status by increasing insulin resistance. The relationship between hyperlipidemia and OSA has not been conclusively established. Overall, cardiovascular risk is increased in untreated OSA pa-tients [5].
Patients with severe OSA in particular develop arterial hypertension with non-dipping (lack of blood pressure reduction overnight). Even patients who have only upper airway obstruction during REM sleep due to the muscle relaxation that occurs there show the symptomatology of arterial hypertension (6). There is a strong association of untreated OSA with cardiac arrhythmias, especially atrial fibrillation [7], cardiac diseases such as CHD and myocardial infarction [8], and the occurrence of stroke [9]. If there is excessive daytime sleepiness due to OSA, an increased cardiovascular risk can also be assumed [10]. Due to the possible occurrence of daytime sleepiness up to excessive daytime sleepiness in the context of OSA, the accident risk of the affected patients is particularly high, especially when occupations with control and monitoring activities are performed. There is no obligation on the part of physicians to notify the patient, but it is mandatory to inform the patient of this circumstance; the information provided should be documented in writing and countersigned by the patient.
By performing cardiorespiratory polygraphy, the severity of OSA can be determined. For this purpose, the apnea-hypopnea index (AHI) of the affected person is determined. The AHI includes the number of apneas and hypopneas per hour of sleep. Mild findings correspond to an AHI of 5-15, moderate findings to an AHI of 15-30/h, and severe findings to an AHI greater than 30/h.
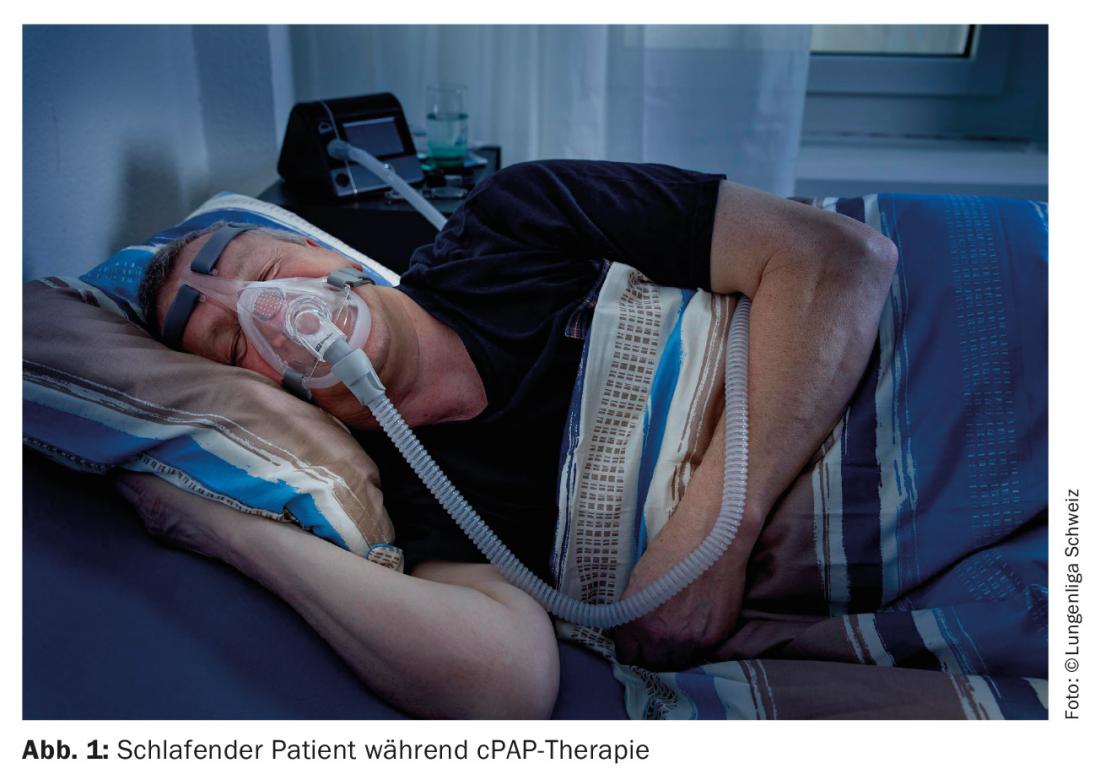
cPAP therapy: The gold standard of therapy for OSA is continuous pneumatic splinting of the upper airway by means of positive airway pressure, applied via a nasal/mouth/nasal mask (Fig. 1). Continuous positive airway pressure (cPAP) therapy is the most commonly used treatment. Pneumatic splinting eliminates upper airway obstruction and thus, ideally, all apneas and hypopneas and, consequently, nocturnal hypoxia. By eliminating hypoxia-induced arousals, the sleep architecture and thus the restfulness of sleep is improved, leading to a reduction in concomitant daytime sleepiness. As a side effect, snoring is significantly eliminated, eliminating what is often cited by the bed partner as socially disruptive, which increases compliance with cPAP therapy. By improving endothelial function [10] and lowering arterial blood pressure [11], cardiovascular risk factors [12] are minimized. Nocturnal positive pressure therapy leads to undesirable side effects in quite a few patients.
A common complaint is morning dryness of the mucous membranes of the upper respiratory tract. This can be remedied by warm air humidifiers, which are nowadays permanently integrated in most cPAP devices and can be individually adjusted by the patient. Nasal obstruction during therapy is described just as frequently, not infrequently leading to discontinuation of therapy at night. The fit of the mask should be checked and changed to another model if necessary. Occasionally, it may be helpful to reduce the positive overpressure of the treatment somewhat, especially to relieve the nasal airway. Reduction of therapy pressure should be monitored during the course at least cardio-respiratory polygraphically, it should not significantly affect the sufficiency of treatment.
Affected patients often independently resort to nasal congestives such as nasal drops or sprays containing xylometazoline to prevent nasal swelling due to increased blood flow to the inferior turbinate. It is known that a habituation occurs here, which gradually leads to a higher frequency of use of the drops/
Sprays leads. In order not to jeopardize cPAP therapy, surgical measures of the inferior turbinate may well be considered. Radiofrequency-assisted conchotomy, the reduction of the inferior turbinate, allows treatments to be performed in a way that is gentle on the tissue and highly successful. Despite the multitude of options for optimizing cPAP therapy, compliance with treatment in the longer term is only 60-70%. In this context, it is helpful to link patients to self-help groups, which can significantly improve the acceptance of treatment among those affected through regular exchange among themselves.
There are some alternatives to cPAP therapy in the treatment of OSA, but their comparative effectiveness is reduced. Mandibular advancement splints (UPS) are the most commonly used, and their comfort has been significantly improved in recent years through the use of new materials and fitting methods. Advancing the mandible reduces oropharyngeal obstruction, especially at the base of the tongue. The prerequisite in the fitting of a UPS is a sufficient dental status, protrusion of the lower jaw and the absence of diseases of the temporomandibular joint. Due to the technical improvements of UPS in recent years, this treatment is now considered equivalent to cPAP therapy in patients with mild-to-moderate OSA if the above-mentioned anatomical-functional requirements are met in the affected individuals.
In severe OSA, UPS should be used only after failure of initialization of cPAP therapy. Typical side effects of UPS treatment are discomfort in the temporomandibular joint, so regular dental or orthodontic check-ups are recommended. In supine-dependent OSA, often in combination with mild-grade OSA, the use of a supine prevention vest may be appropriate. It is not uncommon for these devices to be perceived as uncomfortable to wear at night, so long-term compliance tends to be low. Surgical measures as an alternative to cPAP therapy include uvulopalatopharnygoplasty in combination with tonsillectomy bds. (TE-UPPP) [13], maxillomandibular advancement [14], and hypoglossal nerve stimulation (HGNS). [15] are considered. The indication for one of these surgical procedures requires a precise patient selection with regard to the anatomy and function of the upper airway. The indication for a UPPP-TE requires the presence of hypertrophy of the palatine tonsil bds., which is also found in the distal region at the level of the base of the tongue, as well as a pronounced webbing, i.e., a deep standing of the soft palate with a hypertrophic uvula.
The procedure is characterized by high invasiveness, the possible complications arise not only in the immediate postoperative course, but can also lead to long-term discomfort due to the profound change in the anatomical structure of the oropharynx. It can also be observed that the initial therapeutic success decreases over time, so that UPPP-TE should only be performed after a very strict indication and a very careful patient selection preoperatively. In the presence of mandibular malposition in patients with OSA, maxillomandibular advancement can be considered, whereby osteotomy of the maxilla and mandible achieves advancement of the same and thus the oropharyngeal – especially posterior airway space – obstruction can be removed very effectively.
In principle, patients without skeletal malocclusions of the maxilla/lower jaw are also candidates for this procedure; however, the highly invasive nature of the procedure and the external alteration of the maxillomandibular region of the face should also be considered in this case. HGNS is another surgical alternative for the treatment of OSA. The pacemaker aggregate is implanted analogously to the known cardiac pacemakers, the stimulating electrode of the hypoglossal nerve is placed at the distal end of the nerve, and another electrode for evaluation of respiration is inserted in an intercostal space. As with the other surgical procedures, accurate preoperative diagnostics are required. In particular, a drug-induced sleep endoscopic examination of the upper airway is recommended to rule out concentric obstruction at the level of the oropharynx.
Central sleep apnea
Central sleep apnea (ZSA) is less common than OSA and is characterized by a lack of respiratory effort during the breathing pause, due to dysfunction of the central nervous impulse that controls breathing. In addition to idiopathic occurrence, ZSA occurs in the context of neurological disease patterns due to lesions of the respiratory center located in the medulla oblongata. Respiratory depressant medications, such as opiate use, can also cause ZSA. However, the most common subtype is Cheyne-Stokes respiration, which occurs in up to half of all patients with severe systolic heart failure [16]. Characteristically, spindly crescendo-decrescendo hyperventilation episodes occur between central apneas. Risk factors include male sex, older age, hypocapnia, and the presence of atrial fibrillation [17]. Due to the recurrent hypoxia and the resulting activation of the sympathetic nervous system, malignant cardiac arrhythmias occur frequently in affected patients, which reduce the overall survival of this patient group accordingly [18].
In addition to treatment of the respective underlying disease, an appliance-based treatment approach is possible, as in the treatment of OSA. The cPAP therapy is less effective than in OSA, the appliance-based treatment can be extended by the use of biPAP-ST devices, whereby these devices generate a different pressure level during inspiration and expiration and a minimum breathing frequency is stored; if this minimum frequency is not reached, the devices trigger the next breath. Adaptive servoventilation (ASV) is a special form used in the treatment of Cheyne-Stokes respiration. Echocardiography to determine left ventricular ejection fraction (LVEF) is mandatory before initiating ASV, as ASV should only be used in patients who have an LVEF of >45% [19]. Transvenous phrenic stimulation [20] is another procedure used to treat ZSA. Analogous to HGNS, a pacemaker is implanted which stimulates the phrenic nerve via an electrode; respiration is detected via another electrode in the azygos vein.
Sleep-related hypoxemia and hypoventilation
Sleep-related hypoxemia and hypoventilation are characterized by prolonged periods of ventilatory reduction during sleep, mainly occurring in REM phases with prolonged oxygen desaturations (SB hypoxemia) and concomitant hypercapnia (SB hypoventilation) due to a simultaneous overload of the respiratory pump. Typical is the morning headache that develops due to hypercapnia, accompanied by dizziness, difficulty concentrating and daytime fatigue. In addition to COPD, neuromuscular diseases and thoracic deformities play a significant role in the development of the disease. A special form is obesity hypoventilation syndrome (OHS), which is defined by a hypercapnia of more than 45 mmg/Hg pCO2 during the day and a BMI of more than 30. Many patients have concomitant OSA.
In addition to therapy of the underlying disease, device-based treatment is most often performed using a biPAP-ST device. If the cause is a corresponding clinically relevant COPD, treatment with non-invasive home ventilation (NIV, non-invasive ventilation) [21] is usually required, as well as longer-term oxygen treatment of at least 16h hours daily. Similar to the treatment of OSA by cPAP, the long-term therapeutic success of the above treatment options is correspondingly limited by a lack of compliance on the part of the patients.
Take-Home Messages
- Obstructive sleep apnea (OSA), central sleep apnea (ZSA), and sleep-related hypoxemia and hypoventilation are among the sleep-related breathing disorders (SBAS).
- Excessive snoring combined with pauses in breathing and the resulting daytime sleepiness or drowsiness are typical symptoms of OSA, which is the most common SBAS.
- Heart failure may result in a typical nocturnal breathing pattern, Cheyne-Stokes respiration, which is a form of ZSA.
- Sleep-related hypoxemia and hypoventilation occur in respiratory insufficiency, respiratory pump exhaustion, or impaired pulmonary function.
- In addition to the medical history and clinical examination, cardiorespiratory polygraphy or polysomnography forms the core of the diagnosis.
Literature:
- Fietze I, Laharnar N, Obst A, et al: Prevalence and association analysis of obstructive sleep apnea with gender and age differences – Results of SHIP Trend. J Sleep Res 2019; 28(5): e12770; doi: 10.1111/jsr12770; Epub 2018 Oct 1.
- Stuck BA, Weeß HG: The new “International Classification of Sleep Disorders” – A critical appraisal of the diagnostic criteria for sleep-related breathing disorders. Somnology 2015; 19: 126-132.
- Rodenbeck A: American Academy of Sleep Medicine Manual. Overview of update version 2.0. Somnology 2013; 17: 122-130.
- Schulz R, Eisele HJ, Reichenberger F, Seeger W: Obstructive sleep apnea and metabolic syndrome. Pulmonology 2008; 62(2): 88-91.
- Schulz R, Eisele HJ, Weissmann N, Seeger W: Obstructive sleep apnea: an important cardiovascular risk factor. Deutsches Ärzteblatt 2006; 103: 775-781.
- Aurora RN, Crainiceanu C, Gottlieb DJ, et al: Obstructive sleep apnea during REM sleep and cardiovascular disease. AM J Respir Crit Care Med 2018; 197(5): 653-660; doi: 10.1164/rccm.201706-1112OC.
- Gami AS, Pressman G, Caples SM et al: Association of atrial fibrillation and obstructive sleep apnea. Circulation 2004; 110(4): 364-367; doi: 10.1161/01.CIR.0000136587.68725.8E; Epub 2004 Jul12.
- Gottlieb DJ, Yenokyan G, Newman AB, et al: Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: the sleep heart health study. Circulation 2010; 122(4): 352-360; doi 10.1161/CIRCULATIONHA.109.901801; Epub 2010 Jul 12.
- Arzt M, Young T, Finn L, et al: Association of sleep-disordered breathing and the occurrence of stroke. Am J Respir Crit Care Med 2005; 172(11): 1447-1451; doi: 10.1164/rccm.200505-7020C; Epub 2005 Sept 1.
- Mazzotti DR, Keenan BT, Lim DC, et al: Symptom subtypes of obstructive sleep apnea predict incidence of cardiovascular outcomes. Am J Respir Crit Care Med 2019; 200(4): 493-506; doi: 10.1164/rccm.201808-1509OC.
- Becker HF, Jerrentrup A, Ploch T, et al: Effect of nasal continuous positive airway pressure treatment on blood pressure in patients with obstructive sleep apnea. Circulation 2003; 107(1): 68-73; doi: 10.1161/01.cir.0000042706.47107.7a.
- McEvoy RD, Antic NA, Heeley E, et al: CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med 2016; 375(10): 919-931; doi: 10.1056/NEJMoa1606599.
- Fujita S, Conway WA, Zorick F, Roth T: Surgical correction of anatomic abnormalities in obstructive sleep apnea syndrome: uvulopalatopharyngoplasty. Otolaryngol Head Neck Surg 1981; 89: 923-934.
- Hochban W, Brandenburg U, Peter JH: Surgical treatment of obstructive sleep apnea by maxillomandibular advancement. Sleep 1994; 17: 624-629.
- Strollo PJ Jr, Soose RJ, Maurer JT, et al: Upper-airway stimulation for obstructive sleep apnea. N Engl J Med 2014; 370(2): 139-149; doi: 10.1056/NEJMoa1308659.
- Schulz R, Blau A, Börgel J et al: Sleep apnea in heart failure. Eur Respir J 2007; 29(6): 1201-1205; doi: 10.1183/09031036.00037106.
- Sin DD, Fitzgerald F, Parker JD, et al: Risk facors for central and obstructive sleep apnea in 450 men and women with congestive heart failure. Am J Respir Crit Care Med 1999; 160(4): 1101-1106; doi: 10.1164/ajrccm.160.4.9903020.
- Hanly PJ, Zuberi-Khokhar NS: Increased mortality associated with Cheyne-Stokes respiration in patients with congestive heart failure. Am J Respir Crit Care Med 1996; 153(1): 272-276; doi: 10.1164/ajrccm.153.1.8542128.
- Cowie MR, Woehrle H, Wegscheider K, et al: Adaptive servo-ventilation for central sleep apnea in systolic heart failure. N Engl J Med 2015; 373(12): 1095-1105; doi: 10.1056/NEJMoa1506459.
- Ponikowski P, Javaheri S, Michalkiewicz D, et al: Transvenous phrenic nerve stimulation for the treatment of central sleep apnea in heart failure. Eur Heart J 2012; 33(7): 889-894; doi: 10.1093/eurheartj/ehr298.
- Köhnlein T, Windisch W, Köhler D, et al: Non-invasive positive pressure ventilation for the treatment of severe stable chronic obstructive pulmonary disease: a prospecitve, mulicentre, randomised, controlled clinical trial. Lancet Respir Med 2014; 2(9): 698-705; doi: 10.1016/S2213-2600(14)70153-5.
InFo PNEUMOLOGY & ALLERGOLOGY 2021; 3(3): 11-15.