For a long time, the treatment of lupus was characterized by stagnation. However, after 20 years of frustration, there is finally movement to develop new therapeutic options. Studies have produced promising results for three agents specifically for the treatment of lupus nephritis. The old principle of induction and conservation has finally had its day.
As with all genetic diseases, systemic lupus erythematosus (SLE) has monogenetic forms. But for every patient who has such a bad mutation, there are 1000 others who have minor variants. In those, for example, not the whole protein is missing, but only a single domain, which means that they have a much milder course of the disease. Patients in lupus often have gene variants not just in one gene, but in many, and then ultimately it is the sum of the little things that defines this disease.
This constellation is individually different for each patient. Regarding lupus nephritis (LN), the gene variants also affect the kidney, which may result in, for example, basement membrane weakness (patients quickly develop hematuria), endothelial weakness (leading to vascular complications), or podocyte weakness (proteinuria, nephrotic syndrome). Onset, class, and prognosis of LN are genetically determined. Genetic diagnosis is particularly worthwhile in cases with a family history, childhood SLE, and atypical/syndromic cases.
The widespread assumption that lupus is a disease that occurs in relapses can be rejected, at least for lupus nephritis, explained Prof. Dr. Hans-Joachim Anders, Nephrology Center, LMU Munich [1]: “There is no relapse of LN, there are always only people who do not adhere to their therapy or do so inadequately. Or where the doctor and patient have decided to reduce the dose – and then the disease starts again at some point because the therapy was not sufficient. But it’s a chronic disease that persists.” It is therefore necessary to find the dose of medication that is sufficient and then stick to it.
No LN without CKD
Proteinuria is always also an indication of kidney disease, the nephrologist reminded. “If you have positive protein in the test strip, it almost always means that there is podocyte damage.” Podocytes are the cells of the filtration barrier in the glomeruli that are particularly sensitive, and when they are damaged (immunologically, toxically, or genetically) proteinuria occurs. For this reason, the detection of proteinuria in the urine test strip is always a sign of kidney disease. “You don’t need to send the patient to a urologist in that case; there is no disease in urology that would explain proteinuria.” Rather, the nephrologist is the right contact in such a case. Hematuria, on the other hand, can sometimes occur in urologic diseases, but of course it also occurs in renal diseases. In particular, the combination of hematuria and proteinuria can really only be explained by renal disease. “That’s usually called a nephritic syndrome, and it’s particularly common in lupus.”
However, hematuria allows conclusions to be drawn that a basement membrane must have broken, because otherwise the erythrocytes would not be able to pass through. In this respect, blood in the urine is always a sign of a broken basement membrane, and if it occurs together with proteinuria, probably from the glomeruli, which makes an immunological background likely in lupus. The guidelines state that patients with proteinuria greater than 500 milligrams per day should be referred for renal biopsy. The biopsy is then evaluated using a score that is now almost 20 years old, which Prof. Anders criticized. Initiatives for an update exist, but nothing concrete is available yet.

Important: Any patient who has lupus nephritis has, by definition, chronic kidney disease (CKD). “That’s when the nephrologist should be involved at some point, not when it goes to dialysis.” LN is always present for longer than three months, and that makes it chronic. There are 5 different stages: if kidney function is still ok, LN may well be stage 1, but it is still chronic kidney disease in any case. Kidney disease also means that nephrons are lost faster than through the normal aging process. This shortens kidney life. “And after all my patients want to live at least 90 years, it’s already getting tight in the back,” the expert cautioned. He also reminded the audience that while 10- or 15-year rates sound good to medical professionals, they are of no interest to 25-year-old women, who don’t want to be on dialysis even at 40. In this respect, it is necessary to fight for the patients’ nephrons long before the creatinine rises. When this happens, half of the nephrons have already been lost.
Keep cortisone on a low flame
Prof. Anders referred to the term “refractory lupus nephritis” as an old wives’ tale. It is true that there are already patients who have lupus that is difficult to control immunologically. However, his recommendation was to use standard therapy (steroid pulses plus first line IS therapy) in such cases, to which most patients respond. The guidelines would say to first use cyclophosphamide or MMF in LN and if that doesn’t work to switch, but “that doesn’t make any sense.” The expert compared this to the administration of an antibiotic in the case of an infection, which is not replaced by any other antibiotic if there is no response. Here, as there, one would have to consider re-diagnosis instead, perhaps a smear test. “So if the pat doesn’t respond to first-line therapy, talk to a center or do biopsy, genetic diagnosis, immune profiling.” And most importantly, check for non-adherence now that MMF is increasingly being used orally.
How to increase adherence – generally, as a physician, you should talk a lot with the patient (at least 1 Std. Consultation): Pathogenesis (contagion, heritability), taking the pill, vaccinations, cardiovascular risk factors, reducing anxiety, and avoiding “hocus pocus” such as alternative medicine practitioners or the use of hot stones should be addressed and carried out in depth. Adherence can also be ensured through self-responsibility and self-protection: sun protection (at least strength 50) as early as April is undoubtedly part of this. Hydroxychloroquine can be used in all patients, but not oral cortisone >5 mg (belimumab if necessary). The so called “thrust” – which as explained before does not exist at all – is no longer treated orally with the 20 mg cortisone as it was done in the past. Instead, nowadays there is only 3 times 250 mg methylprednisolone pulse therapy over a period of 3 days. After that, one continues with the normal maintenance dose, otherwise the cortisone exposure is much too high. However, the last mg of cortisone should not be discontinued, the expert advised. “Otherwise, one-third of patients will relapse again.” Lupus is a permanent disease, he said, so therapy must also be used on a permanent basis. 2-4 mg cortisone in the long term does not make much difference.
Hydroxychloroquine must be reduced by 50% if renal function deteriorates, but fortunately only at an eGFR of 20 ml/min (the guidelines recommend at 30 ml/min).
Sunscreen for self protection
Therapeutically, nothing can be changed in the basic problem, the genetic makeup. Only the prevention of cell death is possible. First and foremost, that means slathering on sunscreen. This is because sunburn regularly leads to the release of vast amounts of nuclear material, and this can actually trigger a surge. Therefore, careful sun protection is recommended for all lupus patients.
Rituximab has not really caught on as an option for immunosuppression. Studies show that critically ill patients seem to benefit, but related to all patient groups the p-value is not good enough, therefore all studies so far negative. In contrast, a much more potent B-cell depliter than rituximab is obinutuzumab (OBI), which eliminates all B cells within a few days, which is why it is more potent. Approved in multiple sclerosis, the phase 2 NOBILITY trial has now demonstrated efficacy in LN. Shown was an effect size of 22% across all patients with biopsy-proven LN – corresponding to a doubling of the number of patients who achieved a complete response. Off label obinutuzumab can already be used.
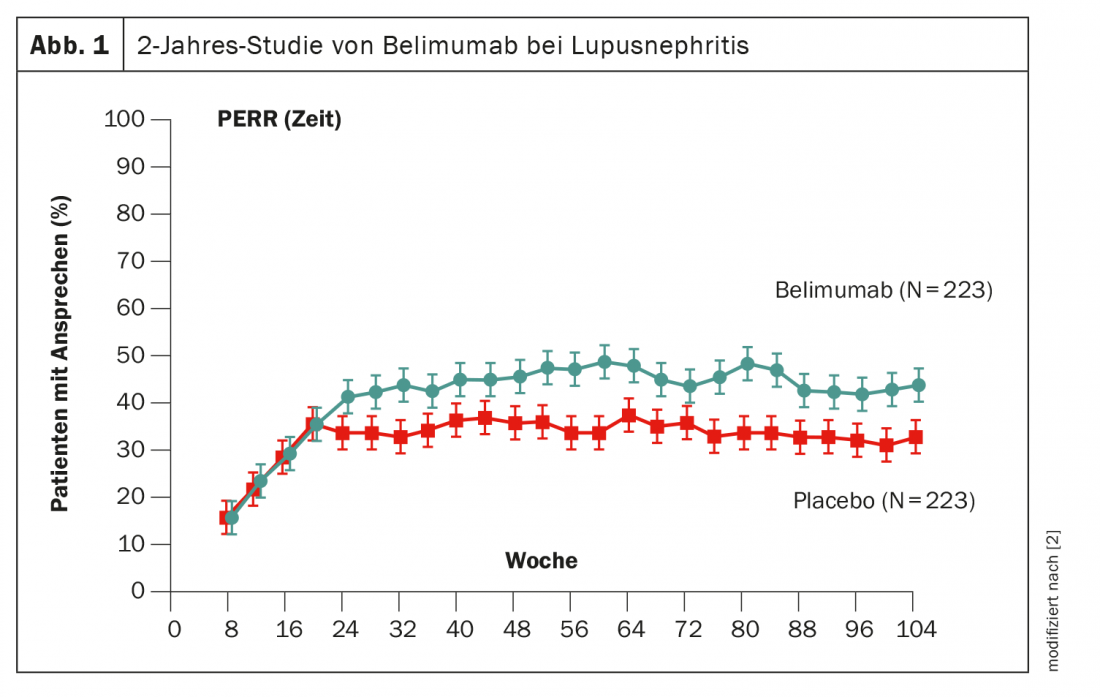
Another way to manipulate B cells is belimumab, which has already been proven in use. In the phase 3 BLISS trial, patients were given belimumab from baseline for 2 years [2]. After approximately 6 months, an advantage over placebo became statistically significant, although the effect size was lower than obinutuzumab at 11% (Fig. 1). The active ingredient is already approved in Switzerland.
Tacrolimus is one of the immunosuppressive drugs that act primarily on T-cell proliferation. The active ingredient has already been discussed in recent years, as it is used in combination with MMF in Asia. However, no data are yet available for Europeans. For voclosporin similar to tacrolimus also a calcineurin inhibitor, the phase 3 AURORA trial has shown efficacy. The effect size after 52 weeks was 18%, and the effect was mainly related to proteinuria. Again, there was almost a doubling of the number of those cases that achieved a complete response.
What all these new therapeutic options have in common is that they no longer aim at induction and maintenance, but suggest long-term combination therapy from the outset. “Lupus is thus recognized as a chronic autoimmune disease that also requires chronic therapy,” Prof. Anders said. “You give a high dose of cortisone at the beginning and then move on to continuous therapy. That seems to work far better than the old ideas of induction and maintenance.”
In monitoring, a lot depends on protinuria: if it goes down to below 0.8 within a year, that has a very high predictive value for long-term outcome, so these patients have a good long-term prognosis. Those who fail to do so may need another protocol or re-biopsy after 12 months. Prof. Anders’ advice to colleagues: “In our case, we explain to all patients at the first biopsy that there will be a second one in a year’s time so we can see if the therapy has worked.”
Summary
- Lupus is a polygenetic disease that results in a loss of tolerance to nuclear material.
- The prognosis is individual.
- Second round diagnostics if no CR
- Therapeutic CYC/MMF and AZA/MMF, new therapeutic options will come/are already available (obinutuzumab, belimumab, voclosporin).
- For monitoring SCr/proteinuria-based nephrology, if patient is unresponsive control biopsy. If not responding, also consider contacting a lupus center.
– FomF Rheumatism Nephro Refresher (online)
Sources:
- “Antisynthetase Syndromes” lecture at FomF Rheumatoid Nephro Refresher (online), 10/30/2020.
- Furie R, et al: Two-Year, Randomized, Controlled Trial of Belimumab in Lupus Nephritis. N Engl J Med 2020; 383: 1117-1128; doi: 10.1056/NEJMoa2001180.
InFo PAIN & GERIATRY 2020; 2(2): 28-30 (published 7/12/20, ahead of print).