Patients with chronic obstructive pulmonary disease (COPD) are susceptible to pulmonary infections, which significantly exacerbate symptoms (especially dyspnea). Epidemiologic data, however, are scarce.
Patients with chronic obstructive pulmonary disease (COPD) are susceptible to pulmonary infections, which significantly exacerbate symptoms, especially dyspnea. However, epidemiological data on such infection-exacerbated COPD (AECOPD) are scarce [1].
COPD (GOLD II-IV) has a prevalence (>40 years) of approximately 5.9%. This patient population each experiences between 0.6-2.7 acute exacerbations per year [2]. Acute respiratory deterioration poses a significant mortality risk to COPD patients; approximately 10% of patients with AECOPD who are hospitalized die. Triggers for AECOPD are in the majority of cases (about 60%) infections, about half viral and half bacterial. In about 30% of cases, no triggering factor can be found. It should be borne in mind that inhaled noxious substances such as nicotine and nitrogen oxides can trigger an exacerbation.
Acute respiratory deterioration can, of course, be triggered by other factors, so that differential diagnoses may include heart failure, pneumothorax, pleural effusion, pulmonary emboli, or new-onset arrhythmias in addition to acute pneumonia [3]. In a study of 1016 patients, the reasons for hospitalization for suspected AECOPD were 48% respiratory infections, 26% heart failure, 3% bronchial carcinoma, 1% pulmonary embolism, and 1% pneumothorax [4]. Therapeutic options during AECOPD are not currently standardized and some are limited in their effectiveness. Therefore, prevention of exacerbation is of great importance.
COPD patients with repeated exacerbations have reduced quality of life and life expectancy (approximately 10% of hospitalized AECOPD patients die). Prevention of exacerbation can be achieved, for example, using oral mycolytics and bronchodilators. It is interesting to note in this context that although the severity of an exacerbation is reduced, mortality is not [5]. The following is a discussion of imaging options in AECOPD. However, a focus will be on diagnostic risk assessment of AECOPD in patients with COPD.
Imaging
Patients with COPD in particular are unable to hold their breath for long periods of time and have difficulty lying flat on their backs. In order to still obtain “sharp” images of the thorax, the image must therefore be acquired as quickly as possible. Two examination modalities fulfill these requirements: X-ray and computed tomography (CT). The PROVIDI trial thoroughly investigated the potential of CT to predict AECOPD [6].
X-ray thorax
The initial radiologic evaluation of a patient with AECODP involves chest radiography, if possible in the standing position in 2 planes [2]. This can be used to rule out differential conditions such as pneumonia, pneumothorax, pleural effusion, or heart failure. In approximately one-fifth of presumptive AECOPD patients, the diagnosis changes, primarily because of pneumonia, and therapy changes accordingly [3,7–9].
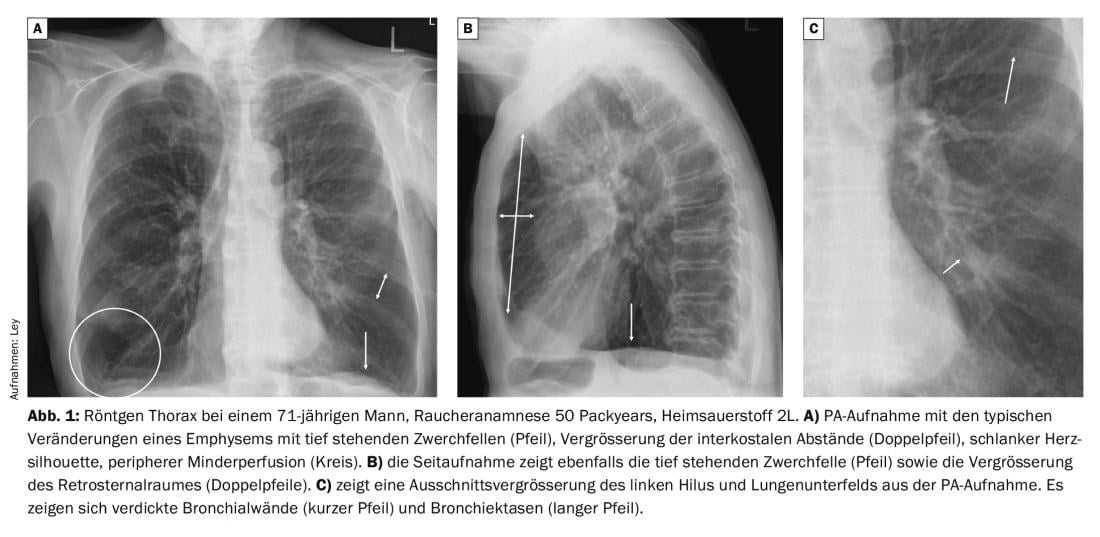
X-rays of the thorax in patients with COPD show characteristic changes compared to healthy individuals. In particular, marked hyperinflation is evident, with low-standing diaphragms, enlargement of the retrosternal space, and enlargement of the intercostal distances. The cardiac silhouette is usually rather narrow and a rarefied pulmonary vascular pattern is seen. In the course of an infection exacerbation, there is often a thickening of the bronchial walls, which leads to a significantly reduced ventilation of the peripherally located lung sections (Fig. 1).
As stated above, relevant findings such as pneumonias are seen in approximately 20% of patients. In the case of a typical, bacterial infection, a two-dimensional compression of a segment, lobe or the entire lung is seen (Fig. 2).

A recent study of non-hospitalized AECOPD patients showed an infiltrate in 20% of cases [10]. Pathogens identified were numerous, e.g., Haemophilus and Streptococcus. Interestingly, there were no differences in pulmonary colonization between COPD patients with exacerbation and those without. On the other hand, pneumonia appeared more frequently in the winter months. Therefore, it was concluded that exacerbations and pneumonias in COPD patients share common infectious triggers and represent a continuum rather than separate entities.
Computed tomography (CT)
CT of the thorax focusing on the lung parenchyma can be performed without intravenous contrast administration. Admission in respiratory failure is desirable but cannot always be realized by patients with AECOPD. In some cases, a flat supine position on the CT examination table is hardly possible, and then holding one’s breath for 4-10 seconds (depending on the CT device) is also an enormous challenge for patients. When it comes to ruling out pulmonary artery embolism, i.v. KM administration is essential. For both questions, 1-mm layers have become established as the layer thickness.
The phenotypic changes of COPD can be classified into an emphysema phenotype and a respiratory phenotype [11]. Bronchial dilation is said to occur when the lumen of the bronchus is 110-150% of the lumen of the accompanying pulmonary artery. More than 150% is called ectasia. Additionally, a lack of tapering into the periphery is found in bronchiectasis. Bronchiectasis can be cylindrical, varicose, and cystic in configuration.
The bronchial wall is assessed in the ratio of inner to outer diameter: If the ratio is 0.5-0.8, it is called mild wall thickening, <0.5 denotes severe wall thickening. In addition, bronchi that are obstructed by mucus are often found in COPD patients with a history of smoking.
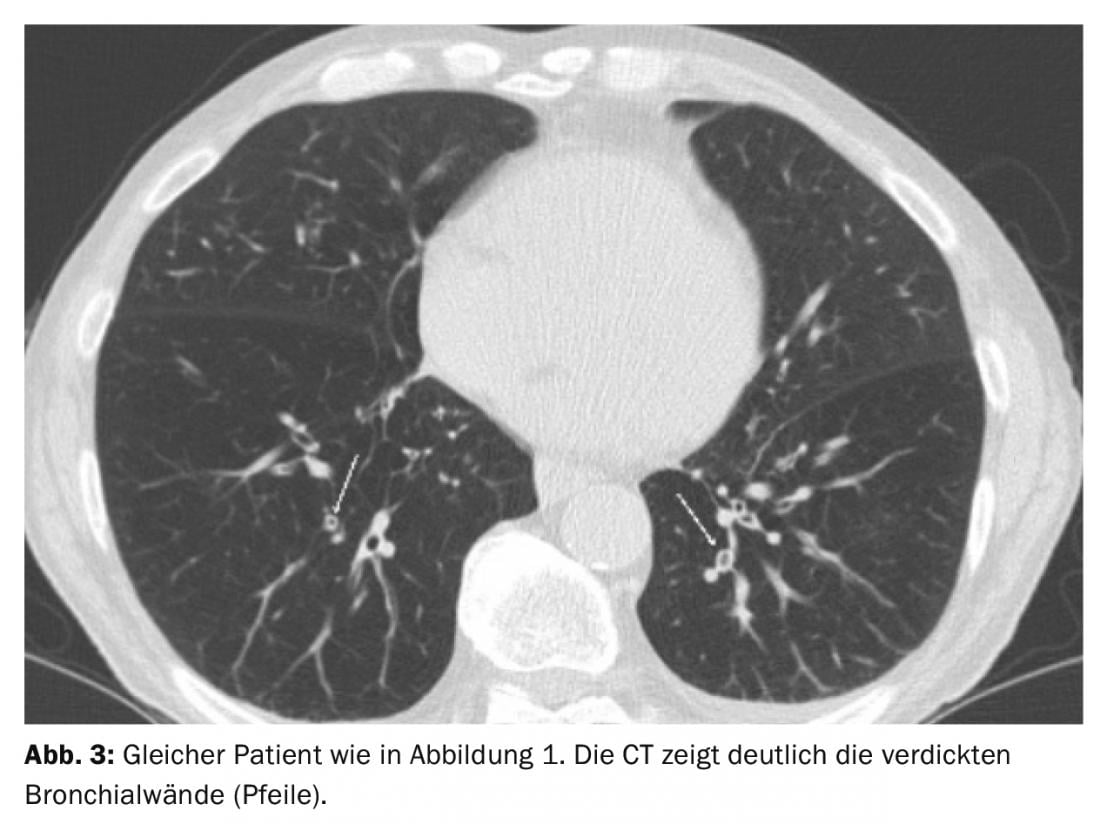
Patients with an airway type appear to be particularly susceptible to AECOPD. Using CT, bronchial wall thickening was shown to occur significantly more frequently in the setting of AECOPD than in the “normal” interval (Fig. 3) [12]. However, interreader agreement for the assessment of bronchial wall thickening is poor.
As mentioned initially, prevention of exacerbation is an important goal. For this, patients with increased susceptibility to exacerbation must be identified. The COPD Gene Study identified 833 patients who had 0-1 exacerbations and 169 patients with more than 2 exacerbations [13]. This showed that for each mm increase in bronchial wall thickness at the segment level, the annual exacerbation rate increased by a factor of 1.84. Patients with more than 35% emphysema showed a 1.18-fold increase in exacerbation rate for every 5% increase in emphysema. These data suggest that routine phenotyping of patients with COPD by CT is reasonable.
In addition to wall thickness, bronchial dilatation is also of great relevance to exacerbation. Bronchiectasis significantly increased the risk of exacerbation (odds ratio 4.99) and was the strongest predictor among several parameters (Fig. 4) [14]. Detection or knowledge of bronchiectasis also has clear therapeutic relevance, as, for example, i.v. antibiosis for P. aeroginosa may be indicated if bronchiectasis is present [1]. The bacterial colonization in such bronchiectasis during exacerbation often suggests atypical pathogens, especially mycobacteria. Here, it was shown that mycobacteria were more frequently detected in patients with infrequent COPD exacerbations than in patients with frequent exacerbations [14].

In addition to the peripheral airways, the central airways, trachea and main bronchi, also play an important role in airflow limitation. Patients with COPD create significant negative pressure in the trachea and main bronchi during inspiration. Two factors (individually or together) lead to expiratory central airway collapse (ECAC) [15].
Over time, softening of the cartilage braces may occur, resulting in malacia. Such tracheobronchomalacia has a prevalence of 5-10% in COPD patients [16]. In addition, there is increased curvature of the pars membranacea. When this results in a lumen reduction of >50%, it is referred to as “excessive dynamic airway collapse” (EDAC) (Fig. 5).
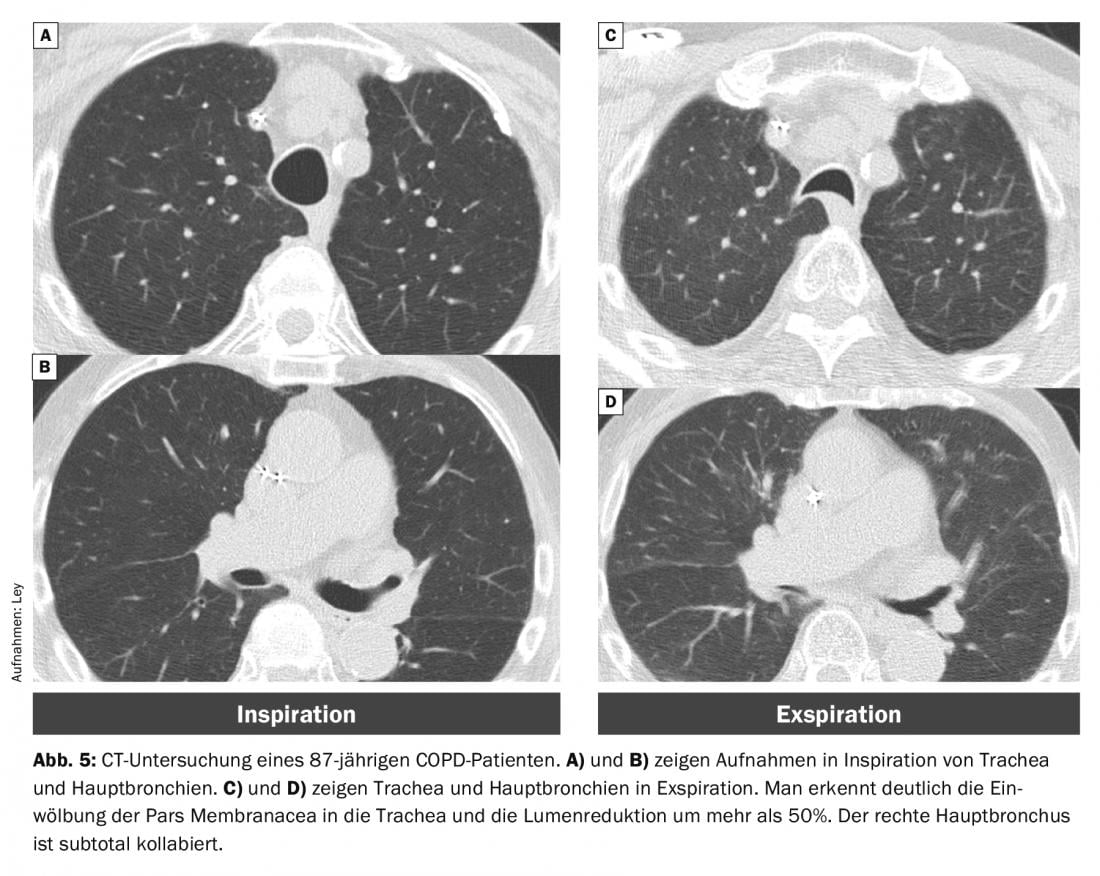
That patients with COPD show significantly higher respiratory collapse than normal patients is well known. However, this does not play a relevant role in the genesis of an exacerbation, as airway instability does not differ between stable and exacerbated COPD [15].
However, there also appears to be an association between the severity of emphysema and exacerbations [17]. Patients were recruited from a lung cancer screening population. COPD phenotype emphysema or non-emphysema type was determined. Emphysema phenotype patients were significantly more severely ill (predicted FEV1: 61% vs. 90%) than non-emphysema COPD patients. It is therefore not really surprising that exacerbations were more frequent in the group of more severely ill patients than in the almost healthy population.
An overarching effect is the ventilation of individual lung regions. Hyperpolarized helium can be used to visualize regional ventilation in magnetic resonance imaging (MRI). Ventilation defects are regularly found in patients with COPD. In patients with mild to moderate COPD, the extent of ventilatory defects was correlated with the number of exacerbations [18]. Ventilatory defects again correlated with the extent of parenchymal destruction (emphysema) and airway disease-thus, a mixed COPD phenotype. Because ventilation MRI examinations can only be performed in single centers worldwide and the assessment of the lung parenchyma/airway is limited, ventilation maps were obtained using CT. For this purpose, inspiratory and expiratory CT datasets from the COPD Gene cohort were examined [19]. Using a non-rigid registration, the data were overlaid. Thus, regional deformation maps could be produced. Surprisingly, patients with increased exacerbations (≥6/year) had more homogeneous ventilation than patients with no exacerbations.
CT datasets also offer the possibility of segmenting anatomical structures, e.g. the airways, due to the high spatial resolution. These segmented data can then be used, for example, to simulate respiratory flows and regional resistances. Forty-two COPD patients were examined by CT during an exacerbation and 6-8 weeks during the course [20]. Airways were segmented from CT data and used to simulate airway flow. This showed that during an exacerbation there is a significant increase in central and peripheral airway resistance. Interestingly, it was mainly the decrease in peripheral airway resistance that was associated with functional recovery. Therefore, excessive inflammation of the peripheral airways appears to be a critical factor in the development of an exacerbation (4th-8th bronchial generation airways were studied). That is, these regions must be reached therapeutically, but because respiratory flows are significantly altered during an exacerbation, oral medications or extra-small diameter inhaled agents must be administered.
Excursus Pulmonary Hypertension
It is known that COPD affects not only the small airways and alveoli, but also the small pulmonary arteries (diameter <500 µm). These vascular changes are also found in patients with moderate COPD and in smokers with normal lung function. Therefore, vasculopathy is likely to occur at early stages of smoker-associated respiratory disease. Approximately 4% of patients with COPD have pulmonary hypertension (PH), and PH-COPD is listed in Group 3 of the WHO classification of PH [21]. In a large study of over 54,000 participants, PH-COPD was a significant risk factor for inpatient AECOPD treatment and mortality. On the other hand, an echocardiographic study demonstrated the effect of exacerbation on right heart function: During exacerbation, the estimated PASP was 40 mmHg and after convalescence, 29 mmHg [22]. However, echocardiography is sometimes difficult in assessing PASP in COPD patients, and the reference standard, invasive right heart catheterization, cannot be performed in all COPD patients. Again, CT helps with a simple measurement: a pulmonary artery diameter to ascending aorta ratio >1:1 was significantly associated with the occurrence of AECOPD (odds ratio 4.78) (Fig. 6) [23]. Specifically, patients in the COPD Gene Study with PA:A ratio >1 had an exacerbation 53% of the time. The diameter determination of the pulmonary artery can be done on axial layers, just before the bifurcation [24].

Take-Home Messages
- When exacerbated COPD is suspected, chest radiography is an important method to identify differential diagnoses.
- Status assessment and characterization of COPD by computed tomography is a useful investigation to determine the individual risk of exacerbation.
- Patients with an airway phenotype of COPD (wall thickening and bronchial dilatation) are especially prone to exacerbation.
Literature:
- Hoffken G, Lorenz J, Kern W, et al: (2005) [S3-guideline on ambulant acquired pneumonia and deep airway infections]. Pulmonology 59: 612-664.
- Lange CG, Scheuerer B, Zabel P: (2004) [Acute exacerbation of COPD]. Internist (Berl) 45: 527-538.
- McCrory DC, Brown C, Gelfand SE, Bach PB: (2001) Management of acute exacerbations of COPD: a summary and appraisal of published evidence. Chest 119: 1190-1209.
- Connors AF, Jr, Dawson NV, Thomas C, et al: (1996) Outcomes following acute exacerbation of severe chronic obstructive pulmonary disease. The SUPPORT investigators Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments). Am J Respir Crit Care Med 154: 959-967.
- Wedzicha JA, Calverley PMA, Albert RK, et al: (2017) Prevention of COPD exacerbations: a European Respiratory Society/American Thoracic Society guideline. Eur Respir J 50.
- Jairam PM, van der Graaf Y, Lammers JW, et al: (2015) Incidental findings on chest CT imaging are associated with increased COPD exacerbations and mortality. Thorax 70: 725-731.
- Emerman CL, Cydulka RK: (1993) Evaluation of high-yield criteria for chest radiography in acute exacerbation of chronic obstructive pulmonary disease. Ann Emerg Med 22: 680-684
- Snow V, Lascher S, Mottur-Pilson C: (2001) The evidence base for management of acute exacerbations of COPD: clinical practice guideline, part 1. Chest 119: 1185-1189.
- Soto FJ, Varkey B: (2003) Evidence-based approach to acute exacerbations of COPD. Curr Opin Pulm Med 9:117-124
- Williams NP, Ostridge K, Devaster JM, et al: (2018) Impact of radiologically stratified exacerbations: insights into pneumonia aetiology in COPD. Respir Res 19: 143.
- Lynch DA, Austin JH, Hogg JC et al (2015) CT-definable subtypes of chronic obstructive pulmonary disease: a statement of the Fleischner Society. Radiology. 10.1148/radiol.2015141579:141579
- Hackx M, Ghaye B, Coche E, et al: (2015) Severe COPD exacerbation: CT features. Copd 12: 38-45.
- Han MK, Kazerooni EA, Lynch DA, et al: (2011) Chronic obstructive pulmonary disease exacerbations in the COPDGene study: associated radiologic phenotypes. Radiology 261: 274-282.
- Kawamatawong T, Onnipa J, Suwatanapongched T (2018) Relationship between the presence of bronchiectasis and acute exacerbation in Thai COPD patients. Int J Chron Obstruct Pulmon Dis 13: 761-769
- Leong P, Tran A, Rangaswamy J, et al: (2017) Expiratory central airway collapse in stable COPD and during exacerbations. Respir Res 18:163
- Patel R, Irugulapati L, Patel V, et al: (2009) The Prevalence of Tracheobronchomalacia in Patients with Asthma or Chronic Obstructive Pulmonary Disease. The Internet Journal of Pulmonary Medicine 12: 1-5.
- Barros MC, Hochhegger B, Altmayer S, et al: (2018) Quantitative computed tomography phenotypes, spirometric parameters, and episodes of exacerbation in heavy smokers: An analysis from South America. PLoS One 13: e0205273.
- Kirby M, Pike D, Coxson HO, et al: (2014) Hyperpolarized (3)He ventilation defects used to predict pulmonary exacerbations in mild to moderate chronic obstructive pulmonary disease. Radiology 273: 887-896.
- Bragman FJ, McClelland JR, Modat M, et al: (2014) Multi-scale analysis of imaging features and its use in the study of COPD exacerbation susceptible phenotypes. Med Image Comput Assist Interv 17: 417-424.
- Hajian B, De Backer J, Vos W, et al: (2018) Changes in ventilation-perfusion during and after a COPD exacerbation: an assessment using fluid dynamic modeling. Int J Chron Obstruct Pulmon Dis 13: 833-842.
- Medrek SK, Sharafkhaneh A, Spiegelman AM, et al: (2017) Admission for COPD Exacerbation Is Associated with the Clinical Diagnosis of Pulmonary Hypertension: Results from a Retrospective Longitudinal Study of a Veteran Population. Copd 14: 484-489.
- Ozben B, Eryuksel E, Tanrikulu AM, et al: (2015) Acute exacerbation impairs right ventricular function in COPD patients. Hellenic J Cardiol 56: 324-331.
- Wells JM, Washko GR, Han MK, et al: (2012) Pulmonary arterial enlargement and acute exacerbations of COPD. N Engl J Med 367: 913-921.
- Rho JY, Lynch DA, Suh YJ, et al: (2018) CT measurements of central pulmonary vasculature as predictors of severe exacerbation in COPD. Medicine (Baltimore) 97: e9542.
- Vogelmeier C, Buhl R, Burghuber O, et al: (2018) S2k guideline on diagnosis and treatment of patients with chronic obstructive bronchitis and emphysema (COPD). AWMF online.
InFo PNEUMOLOGY & ALLERGOLOGY 2019; 1(2): 10-14.