Medical management of malignant brain tumors, especially malignant gliomas, is focused on palliation because of poor survival and concomitant morbidity. Vasogenic cerebral edema is treated with corticosteroids, and dexamethasone is the standard therapeutic agent. Dexamethasone is usually prescribed too often, for too long, and in too high a dose. The lowest effective dose should be titrated in consultation with the patient and family members. Prophylactic anticonvulsant therapy in the absence of epileptic seizures is not indicated. Important aspects of the terminal phase should still be discussed with all parties involved in the phase of preserved communication and recorded with an advance directive.
Brain tumors of the CNS are divided into low-grade (WHO grades I-II) and higher-grade gliomas (WHO grades III-IV). Among them are astrocytomas, oligodendrogliomas and their mixed forms, and much less frequently the ependymomas and other variants [1].
Malignant gliomas are characterized by rapid tumor growth and the appearance of necrosis. These occur when the vascular supply to the tumor can no longer be guaranteed. In response to the deficiency, vascular cytokines and growth factors (including vascular endothelial growth factor, VEGF) are produced by both neoplastic and host cells to restore tissue homeostasis [2]. Rapidly growing tumor-supplying vessels are immature and permeable. As a result, hemorrhage develops in the tumor and peritumoral vasogenic cerebral edema regularly develops. In addition to destruction of neurons and supporting tissue by malignant infiltration, brain edema is a major factor in morbidity and mortality in gliomas.
Characteristically, malignant cells obtain their energy through anaerobic glycolysis [3]. The resulting lactate acidifies the surrounding environment, so that chemotherapeutic agents, anticonvulsants and other drugs can penetrate poorly into the tumor regions. The additional formation of excitatory neurotransmitters such as glutamate interferes with the function of neurons in the peritumoral region and provokes neurological symptoms and epileptic seizures [4].
Initiating palliative care as early as possible
Progress has been made in recent years in multimodal initial therapy consisting of maximal possible resection, postoperative radiochemotherapy, and systemic maintenance and recurrent therapy. Nevertheless, medical management, especially for malignant gliomas, is focused on palliation because of poor survival and concomitant morbidity. Therefore, the drug and non-drug principles of palliative care should be incorporated into the therapeutic concept at an early stage [5]. The aim of therapeutic efforts must be to preserve the patient’s quality of life and autonomy as much as possible. This includes prophylaxis and treatment of so-called therapeutic and glioma-associated toxicity.
In the following, the drug treatment of cerebral edema and epileptic seizures as well as the management of coagulation disorders in brain-derived tumors are presented from a pragmatic point of view.
Perifocal cerebral edema
Vasogenic cerebral edema is non-specific as a CNS response to a growing intracerebral process and occurs in both malignant processes (gliomas, brain metastases) and inflammation (abscesses, autoimmune diseases). Especially in malignant gliomas, the breakdown of the blood-brain barrier is due to the rapid growth of immature tumor vessels [2]. This is the anatomical prerequisite for the leakage of intravenously applied contrast medium into the brain tumor. Tumor growth and vasogenic edema initially increase localized intracranial pressure, displacing surrounding brain matter and causing focal neurologic symptoms. If intracranial pressure increases, midline shift and/or life-threatening displacement of the temporal lobes through the tentorium caudally develops (transtemporal herniation) (Fig.1). The increase in pressure can only be compensated by reducing the intravascular, initially venous, but later also arterial blood volume and cerebrospinal fluid until the physiological reserves are exhausted. Venous outflow congestion and hemorrhage, arterial inferior perfusion, and cerebrospinal fluid circulation disorders, in turn, exacerbate the disease process.
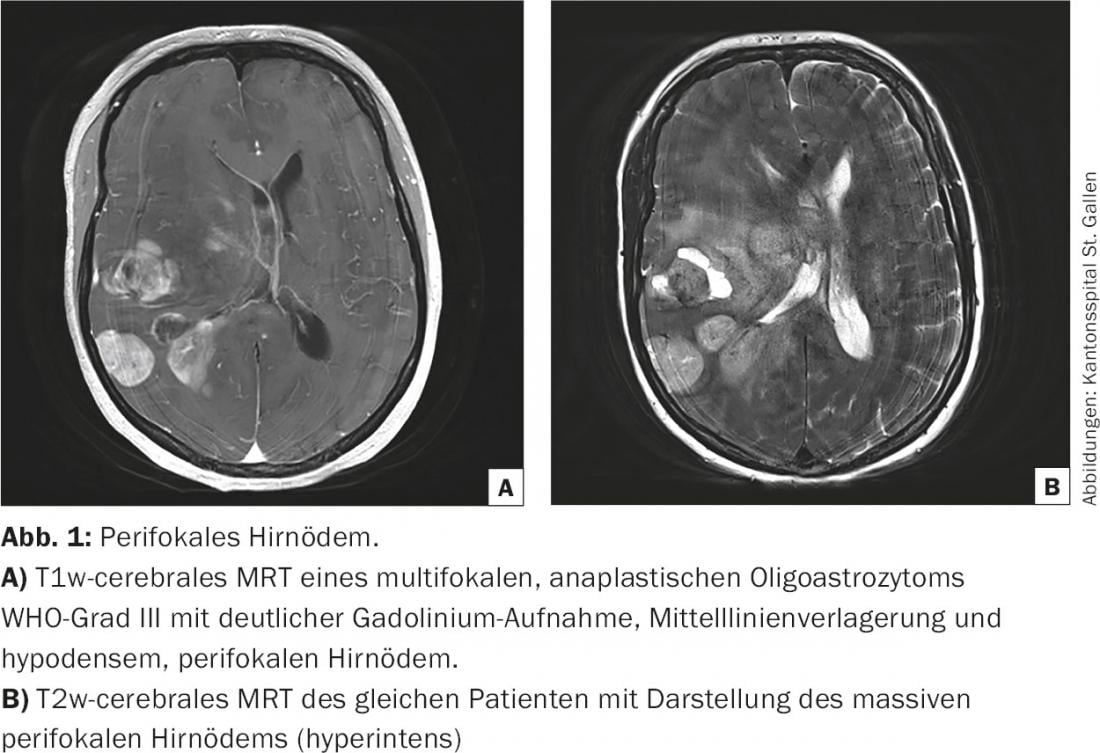
Peritumoral brain edema plays an important role both at initial diagnosis and during the course of brain tumor disease. Often, the initial neurologic disturbances are caused only by the vasogenic cerebral edema and are rapidly reversible (within 24 hours) with the administration of corticosteroids [6]. In this context, steroids are used as a diagnostic therapeutic agent to distinguish a functional disorder (pressure-related) from structural damage (infiltration, destruction). This helps in weighing the risk-benefit of neurosurgery, especially in the recurrence situation. In the terminal phase of brain tumor disease, the increase in intracranial pressure leads to progressive opacification and finally to the death of the patient. This dying process is predominantly peaceful [7].
Management of vasogenic cerebral edema.
In contrast to cytotoxic cerebral edema, which is usually hypoxic, vasogenic cerebral edema is treated with corticosteroids. Dexamethasone is widely used as a standard therapeutic substance in clinical practice. It is characterized by a long biological half-life, can be administered orally and intravenously, and provides rapid symptom relief. The effect is unfolded through modulation of VEGF expression, anti-inflammatory effects and inhibition of arachidonic acid cascade [8]. However, significant side effects can be expected with prolonged therapy, for example, invalidating proximal steroid myopathy, hyperglycemia, hypalbuminemia, electrolyte disturbances, immunosuppression, psychiatric disorders, osteoporosis, and skin hemorrhages [8]. Therefore, careful handling of this drug in brain tumor treatment is critical to avoid substantially compromising the patient’s health status through iatrogenic interventions(Table 1).

Our experience shows that dexamethasone is prescribed too often, for too long, and in too high doses. We recommend that the lowest effective dose be titrated in consultation with the patient and relatives, even at the risk that neurological symptoms may temporarily worsen (tab. 2 and 3) . Due to the long biological half-life, there is no rational argument to apply dexamethasone several times a day. A single dose in the morning is sufficient. Morning administration is better tolerated (sleep disturbances, nocturnal delirium), reflects physiologic release of endogenous corticosteroids, and increases drug adherence. Lipophilic steroids have a critical interaction potential with chemotherapeutic agents, anticonvulsants, and anticoagulants that is too rarely considered.

Epileptic seizures
In 20-40% of brain tumor patients, epileptic seizures lead to the diagnosis of an intracranial mass. One third of all brain tumor patients suffer from structural epilepsy, with low-grade gliomas and neurogenic tumors (e.g., gangliogliomas) being more epileptogenic than malignant gliomas or metastases [9]. Nevertheless, prophylactic anticonvulsant therapy is not indicated [10].
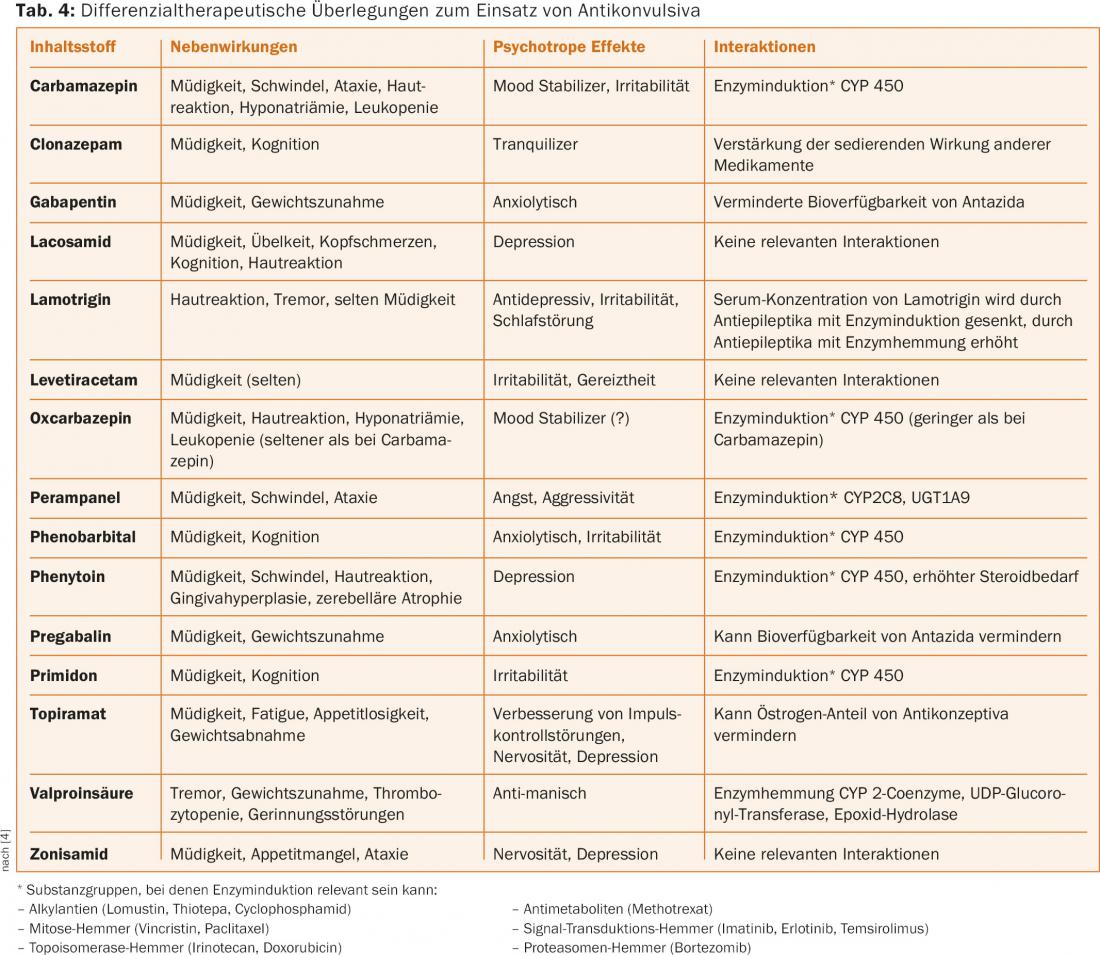
Epileptic seizures affect quality of life by increasing the likelihood that seizures will occur unpredictably despite adequate anticonvulsant therapy. This has consequences for patient autonomy (fear of seizures), restricts mobility (driving ban), and can lead to injury from falls. Education of affected individuals is important in this situation to counteract the usually inflated fear of epileptic seizures. Regular intake of anticonvulsants in therapeutic doses, selection of the optimal drug for differential therapy, and attention to contraindications and interactions (www.cancerdrugs.ch) are important determinants of therapy [10]. A differential therapeutic design for the use of anticonvulsants can be found in table 4. Avoidance of seizure-provoking factors such as sleep deprivation or excessive alcohol consumption as well as good medication adherence are part of this on the patient side. Drugs that lower the seizure threshold (e.g., bupropion, clozapine, and beta-lactam antibiotics) and excessive hyperglycemia should be avoided.
Education about seizures, which are usually self-limiting, and prescription of rapidly acting emergency anticonvulsants (Rivotril, Lorazepam, Dormicum) contribute to patient autonomy and reduction of anxiety. Equally important are instructions on what to do in the event of an epileptic seizure (treatment plan, telephone list) and what activities should be avoided (climbing trees, climbing ladders, bathing alone, changing infants on a changing table instead of on the floor). Not every epileptic seizure requires a visit to the emergency department, where the patient has to wait a long time and unnecessary imaging is ordered. Often, a telephone consultation with a primary care physician or with a neurology service physician is sufficient to manage the acute situation. However, unusual seizures, long seizure duration, prolonged unconsciousness, or postictal agitation should prompt medical consultation to avoid overlooking tumor hemorrhage, tumor progression, or metabolic disturbances (hypoglycemia, electrolyte imbalances).
Coagulation disorders
The risk of postoperative venous thromboembolism in brain tumor patients in the first year is a cumulative 30% [11]. Risk factors include malignant brain tumors, older age, hemiparesis, large tumor volume, and partial tumor resection. As a remote paraneoplastic effect, malignant gliomas secrete vasoactive substances (VEGF, tissue factor) that trigger coagulation disorders such as thrombosis and pulmonary embolism [12]. In the risk assessment, prophylaxis and treatment of thromboembolic events is unrestricted because of the low intracerebral hemorrhage risk (approximately 2%) [13]. Therapeutically, compression stockings and low molecular weight heparins are used in the immediate postoperative period. When venous thromboembolism is detected, parenteral or oral anticoagulation is also indicated in brain tumor patients [11]. This also applies to patients on antiangiogenic therapy with bevacizumab [14].
Special problems at the end of life
Patients with brain tumors differ from other tumor patients in the end-of-life phase by the appearance of specific neurological symptoms [15]. These include swallowing problems, impaired consciousness, progressive neurological deficits, incontinence and headaches.
In particular, impaired communication and neurocognitive disorders interfere with autonomy at the end of life and push the general concepts of palliative care, which are often based on active expression of will, to their limits. Therefore, the important aspects of the terminal phase should still be discussed with all parties involved during the phase of received communication and recorded with an advance directive. These aspects include medical decisions such as artificial feeding versus fasting for death, resuscitation measures, pain management, and sedation, as well as spiritual needs, the place of death, funeral arrangements, and the organization of palliative care [5,16,17].
In case of loss of consciousness, one should critically consider the sense of continued drug therapy by application of fluids, corticosteroids, anticonvulsants, and thrombosis prophylaxis from the aspect of prolonging the suffering [7].
Literature:
- Louis DN, et al: The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 2007; 114: 97-109.
- Wick W, et al: Current status and future directions of anti-angiogenic therapy for gliomas. Neuro Oncol 2016; 18: 315-328.
- Woolf EC, Scheck AC: The ketogenic diet for the treatment of malignant glioma. J Lipid Res 2015; 56: 5-10.
- Hundsberger T, et al: Neurological complications in cancer patients. Praxis 2014; 103: 1009-1016.
- Pace A, et al: Supportive care in neurooncology. Curr Opin Oncol 2010; 22: 621-626.
- Wolfson AH, et al: The role of steroids in the management of metastatic carcinoma to the brain. A pilot prospective trial. Am J Clin Oncol 1994; 17: 234-238.
- Bausewein C, et al: How do patients with primary brain tumours die? Palliat Med 2003; 17: 558-559.
- Roth P, et al: Tumor-associated edema in brain cancer patients: pathogenesis and management. Expert Rev Anticancer Ther 2013; 13: 1319-1325.
- van Breemen MS, et al: Epilepsy in patients with brain tumours: epidemiology, mechanisms, and management. Lancet Neurol 2007; 6: 421-430.
- Rossetti AO, Stupp R: Correlation of enzyme-inducing anticonvulsant use with outcome of patients with glioblastoma. Neurology 2010; 74: 1329-1330.
- Perry JR, et al: Phase II trial of continuous dose-intense temozolomide in recurrent malignant glioma: RESCUE study. J Clin Oncol 2010; 28: 2051-2057.
- Jenkins EO, et al: Venous thromboembolism in malignant gliomas. J of Thrombosis and Haemostasis 2010; 8: 221-227.
- Pan E, et al: Retrospective Study of Venous Thromboembolic and Intracerebral Hemorrhagic Events in Glioblastoma Patients. Anticancer Research 2009; 29: 4309-4313.
- Nghiemphu PL, et al: Bevacizumab and chemotherapy for recurrent glioblastoma: a single-institution experience. Neurology 2009; 72: 1217-1222.
- Sizoo EM, et al: Symptoms and problems in the end-of-life phase of high-grade glioma patients. Neuro Oncol 2010; 12: 1162-1166.
- Koekkoek JA, et al: Symptoms and medication management in the end of life phase of high-grade glioma patients. J Neurooncol 2014; 120: 589-595.
- Pace A, et al: End of life issues in brain tumor patients. J Neurooncol 2009; 91: 39-43.
- Kaal EC, Vecht CJ: The management of brain edema in brain tumors. Curr Opin Oncol 2004; 16(6): 593-600.
InFo NEUROLOGY & PSYCHIATRY 2016; 14(4): 22-28.