The treatment of pityriasis rubra pilaris can be a challenge. The therapeutic arsenal includes topical corticosteroids, phototherapy or classic systemic therapies such as retinoids or immunosuppressants. Recent case reports and smaller case series indicate that the off-label use of biologics is a treatment option to be considered in cases that do not respond to conventional therapy.
The treatment of pityriasis rubra pilaris (PRP) (box) is often difficult and lengthy. A universal treatment approach for PRP does not yet exist. In addition to topical preparations to alleviate symptoms, systemic therapy is used to inhibit inflammation, with both strands of therapy often being combined. In the recent past, several case reports and smaller case series have been published in which biologics led to successful treatment in patients who were difficult to treat. The following is a compact summary of four cases from everyday practice.
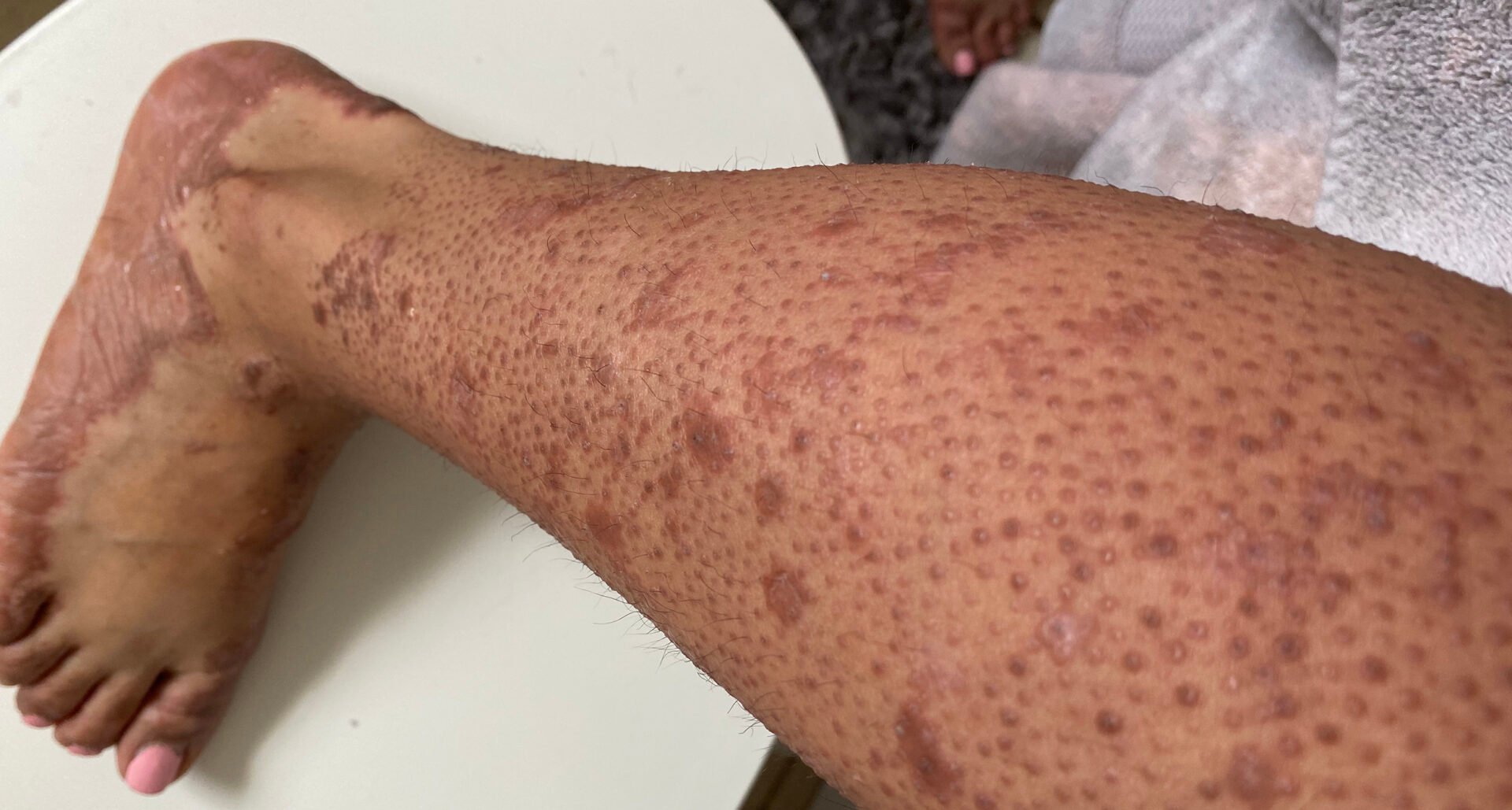
| Pityriasis rubra pilaris (PRP) is an inflammatory erythemato-squamous dermatosis of unknown etiology, which is diagnosed based on classic clinical and histopathologic characteristics. At least 6 subtypes are known to occur in both children and adults [3]. Characteristic features are follicular papules and small, scaly, orange-reddish plaques. PRP usually spreads from the face in a craniocaudal direction, leaving clinically normal skin islands (nappes claires) on the trunk and extremities, including the palms of the hands and soles of the feet [4]. To date, it has not been possible to identify diagnostically relevant serological or immunohistochemical markers. The differential diagnosis varies depending on the PRP subtype. In general, PRP should be distinguished from other papulosquamous diseases, especially psoriasis. |
Ustekinumab for PRP in infants and adults
At this year’s DDG conference, two case reports were presented in which the off-label use of ustekinumab proved to be effective [1]. Ustekinumab neutralizes the interleukins IL-12 and IL-23 and thus has an immunosuppressive and anti-inflammatory effect. In case 1 , Almeida et al. of a six-month-old infant with erythematous-squamous lesions that started on the face and developed into erythroderma [1]. Histology revealed psoriasiform dermatitis. A mutation in the CARD-14 gene was identified (c.349 +2T>C), whereupon the clinical picture was classified as CARD-14-associated papulosquamous eruption (CAPE). The infant showed no response to treatment with methotrexate and acitretin. However, an excellent response was achieved with the weight-adapted administration of ustekinumab.
In case 2, the same study authors described an adult with PRP, which manifested as extensive erythematous-squamous lesions and follicular accentuation [1]. Here too, histology revealed psoriasiform dermatitis. The patient responded neither to methotrexate nor to oral retinoids (isotretinoin), but an improvement in the findings was achieved using ustekinumab.
Risankizumab in long-term PRP patient over 55
Case 3 was presented at last year’s DDG conference [2]. This is a case study in which treatment with the IL-23 inhibitor risankizumab proved to be effective in a patient in middle adulthood. The 57-year-old female patient, who had been suffering from pityriasis rubra pilaris (PRP), type II according to Griffith, for 38 years, had been treated with topical glucocorticosteroids and tacrolimus for several years in the past, but this did not bring about any significant clinical improvement. Systemic therapy with retinoids was sine effectu and caused severe side effects. The patient refused further system therapies.
After detailed clarification, off-label therapy with the IL-23 antibody Risankizumab was initiated at the dosage approved for plaque psoriasis (150 mg s.c. in weeks 0, 4, then every 12 weeks). In addition, pre-existing topical therapy with Tacrolimus 0.1% was continued once or twice daily. Before systemic therapy was initiated, large, confluent, erythematous, partly lichenified plaques with fine lamellar, whitish scaling appeared on the entire integument. In between were islands of healthy skin (nappes claires). The facial area showed extensive erythema with scaling and ectropion on both sides. The fingernails and toenails were dystrophic distally and showed longitudinal furrowing. Pruritus or pain were denied. No concomitant or previous illnesses were known. Just four weeks after the first administration of risankizumab, there was a clear regression of the skin findings with a reduction in the erythematous plaques and almost complete absence of desquamation as well as less pronounced ectropion in both eyes.
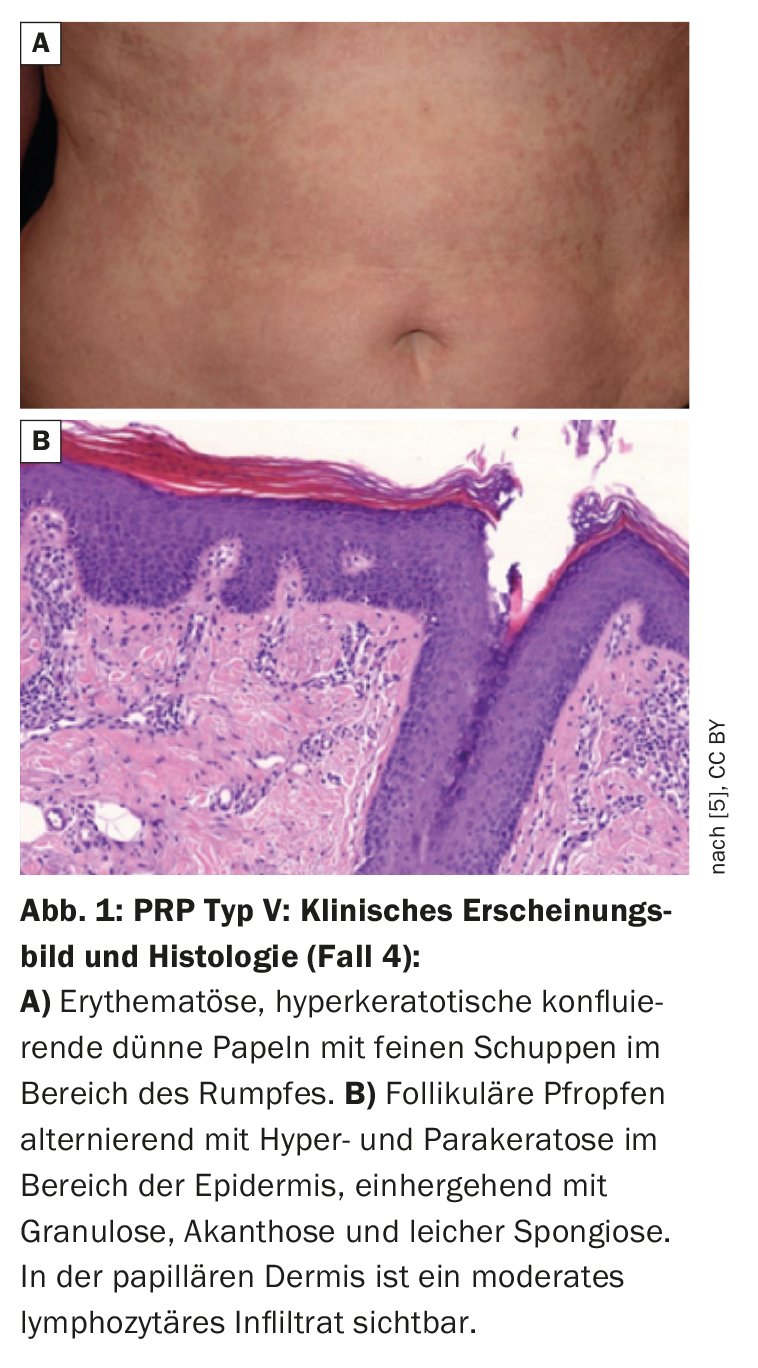
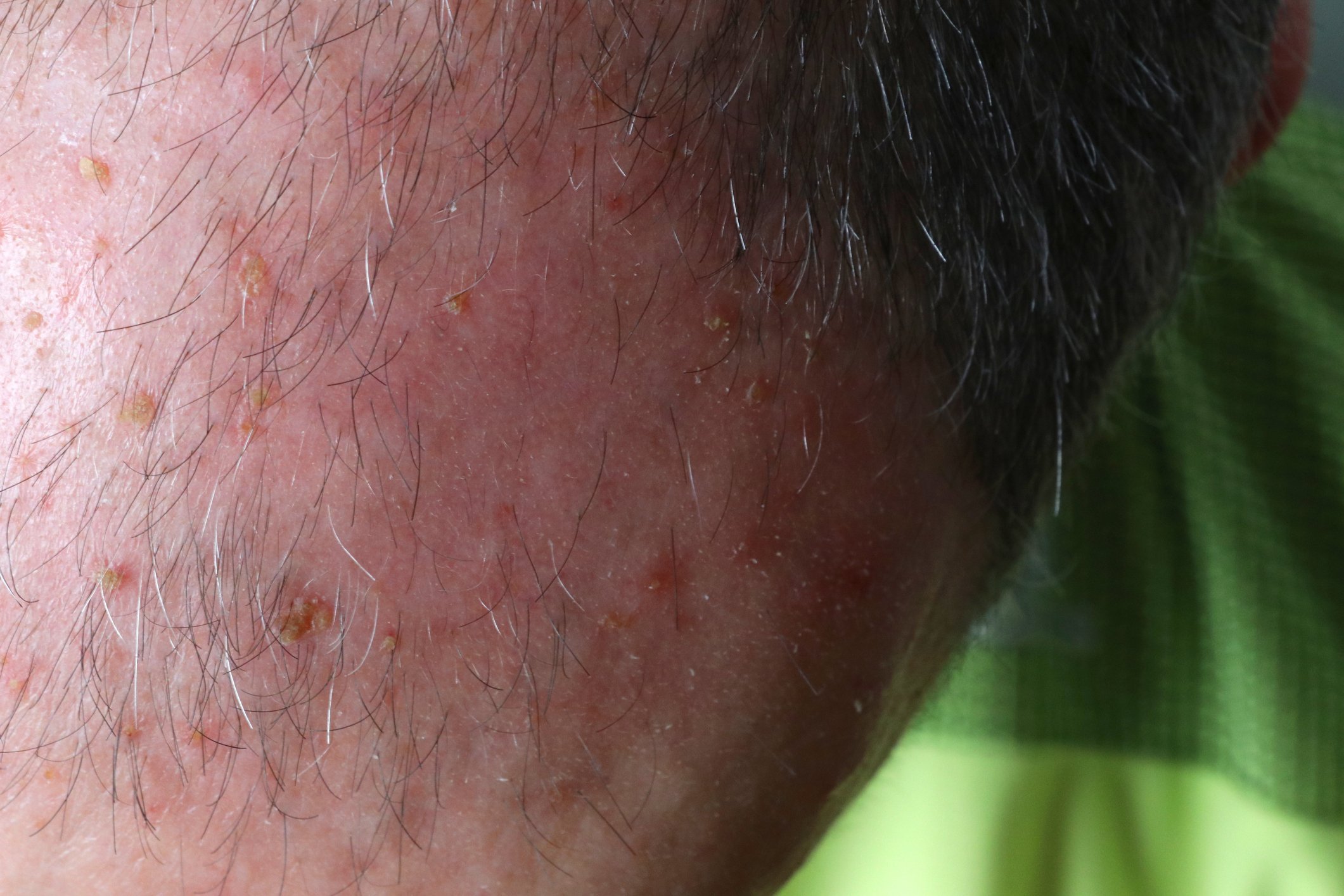
| Case 4: 61-year-old female patient with type V PRP and CARD-14 variants The woman had generalized erythroderma with slight infiltration and whitish fine scales on the trunk (Fig. 1). The skin symptoms first appeared in childhood and the patient had been undergoing dermatological treatment for 28 years. A family history was found – her daughter and a grandchild had psoriasis. Genetic analyses identified CARD-14 variants in the PRP patient. Direct sequencing of the regions coding for CARD-14 led to the identification of three heterozygous missense variants: – c.1641G/C p.Arg547Ser (rs2066964) on exon 14 – c.2044C/T and p.Arg682Trp (rs117918077) on exon 17 – c.2458C/T p.Arg820Trp (rs11652075) on exon 20 Pathogenicity prediction analyses using an appropriate tool showed that the p.Arg682Trp missense variant is pathogenic, while the other two variants are benign. |
| according to [5] |
Association between CARD-14 gene variants and PRP
Although the clinical and histological characteristics of PRP and psoriasis differ, there are nevertheless overlaps between the two clinical pictures, for example in the activation of nuclear factor-kappa B (NF-κB) [3]. Under physiological conditions, this signaling cascade is required to maintain an immunological balance in the skin, but when (over)activated it plays a central role in inflammatory skin diseases [3]. The CARD-14 protein, which is preferentially present in keratinocytes and endothelia of the skin vessels, activates NF-κB [4]. CARD-14-associated papulosquamous eruptions (CAPE) are associated with psoriasis, but also with the atypical juvenile type of pityriasis rubra pilaris (type V) [5–7]. It is assumed that certain CARD-14 mutations cause inflammatory reactions that deviate from the norm and thus contribute to the etiopathogenesis of PRP [5]. Among other things, it has been shown that excessive CARD-14 expression activates the IL-23/Th17 pathway [6], which may explain the treatment response to ustekinumab and risankizumab described in the case studies presented. Figure 1 shows findings of PRP type V with heterozygous missense variants in CARD-14 coding regions (box) [5].
Literature:
- De Almeida H, et al: Successful treatment with ustekinumab in two cases of pytiriasis rubra pilaris spectrum, P095. DDG Conference 2023, volume of abstracts, JDDG 2023; 21 Suppl 1: 1-177.
- Bätcher L, Homey B, Meller S: Clinical control of pityriasis rubra pilaris by risankizumab, P002. DDG Conference 2023, Abstract volume, JDDG 2022; Volume 20 (S1); 1-49.
- Brown F, Badri T. Pityriasis Rubra Pilaris. [Updated 2023 Jun 26]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing.
- Volc-Platzer B: CARD14 mutations in pityriasis rubra pilaris and the treatment response to ustekinumab – a hypothesis. JDDG 2020, DOI: 10.1111/ddg.14218_g.
- Danis J, et al: Nuclear Factor κB Activation in a Type V Pityriasis Rubra Pilaris Patient Harboring Multiple CARD14 Variants. Front Immunol 2018; 9:1564. doi: 10.3389/fimmu.2018.01564, www.frontiersin.org/articles/10.3389/fimmu.2018.01564/full,(last accessed 17.10.2023)
- Frare CP, et al: CARD14-associated papulosquamous eruption (CAPE) in pediatric patients: Three additional cases and review of the literature. Pediatr Dermatol 2021; 38(5): 1237-1242.
- Fuchs-Telem D, et al: Familial pityriasis rubra pilaris is caused by mutations in CARD14. Am J Hum Genet 2012; 91: 163-170.
DERMATOLOGY PRACTICE 2023; 33(5): 48-49
Cover picture: Kelly McGauran, wikimedia