Spontaneous intracerebral hemorrhage (ICB) accounts for approximately 15-20% of all strokes. Although a large proportion of ICB can be treated conservatively, surgical treatment has a high priority. Surgical therapy subsets include intracranial pressure (ICP) control, hydrocephalus and intraventricular hemorrhage management, and surgical hematoma evacuation. Subgroup analyses have been performed for the following situations resp. patient groups demonstrated a benefit of early surgical hematoma evacuation: for symptomatic cerebellar hemorrhage, to reduce mortality in selected patients with severe cerebral hemorrhage, and for atypical near-surface hemorrhage.
The optimal treatment of intracerebral hemorrhage (ICB) remains a controversial and clinically challenging topic even after review of newly published clinical data from randomized prospective trials over the past 15 years.
In practice, the question often arises as to the possibility, necessity and usefulness of an operation. The cooperation of the clinical disciplines involved in neurovascular centers allows the chances of success and risks of an intervention to be weighed up in each individual case. Nevertheless, as a basis for this pragmatic “pros and cons” consideration, the current scientific data situation is also of primary importance. This results in the surgical indication, taking into account ethical aspects, patient-related risk factors, and an assessment of the expected mortality or morbidity. In the evaluation of patients with ICB, age, state of consciousness, and extent and localization of hemorrhage are usually the primary parameters in studies. This article summarizes the most important findings of the current studies in a practically relevant way.
Frequency of intracerebral hemorrhage
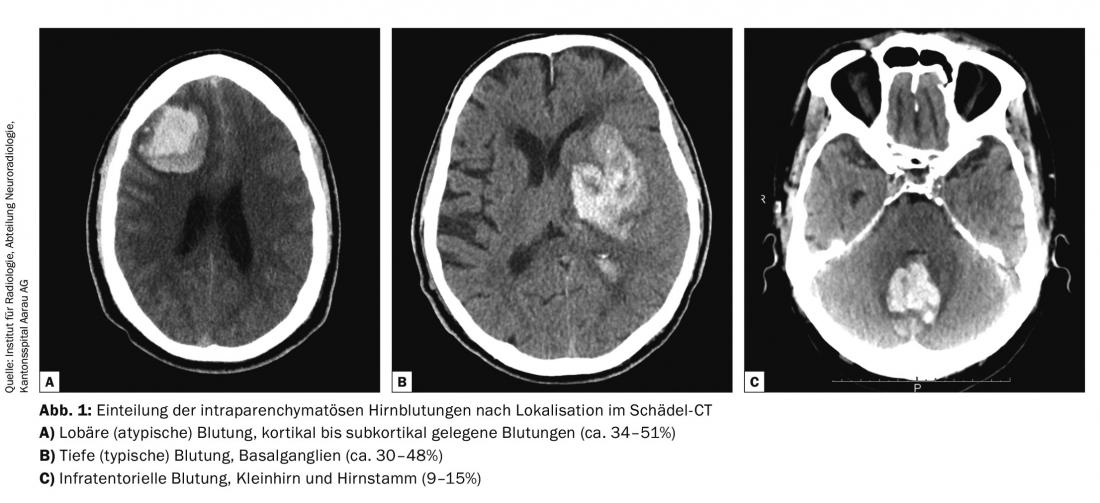
Spontaneous ICB account for approximately 15-20% of all strokes. About half of these are aneurysmal subarachnoid hemorrhage (aSAB), and the other half are spontaneous intraparenchymal hemorrhage. Parenchymal hemorrhages are further divided into three therapy-relevant categories: lobar/superficial hemorrhages (34-52%), deep trunk ganglion hemorrhages (30-48%), and infratentorial/cerebellar hemorrhages (9-15% ) (Fig. 1).
Diagnostics for intracerebral hemorrhage
History and clinical examination are critical in the emergency situation to assess which patients need to be assigned to a care center as soon as possible. An aSAB is usually accompanied by the most severe, “never experienced before” headache. Intraparenchymal hemorrhage presents similarly, often accompanied by neurologic deficits, whereas ischemic stroke itself is often painless and neurologic symptoms lead to the diagnosis here. Therefore, when a stroke is clinically suspected, diagnostic imaging using computed tomography (CT) is indicated as the first diagnostic step. If blood thinning is initiated in a patient with suspected stroke before hemorrhage has been ruled out, there is a risk of further extension of hemorrhage.
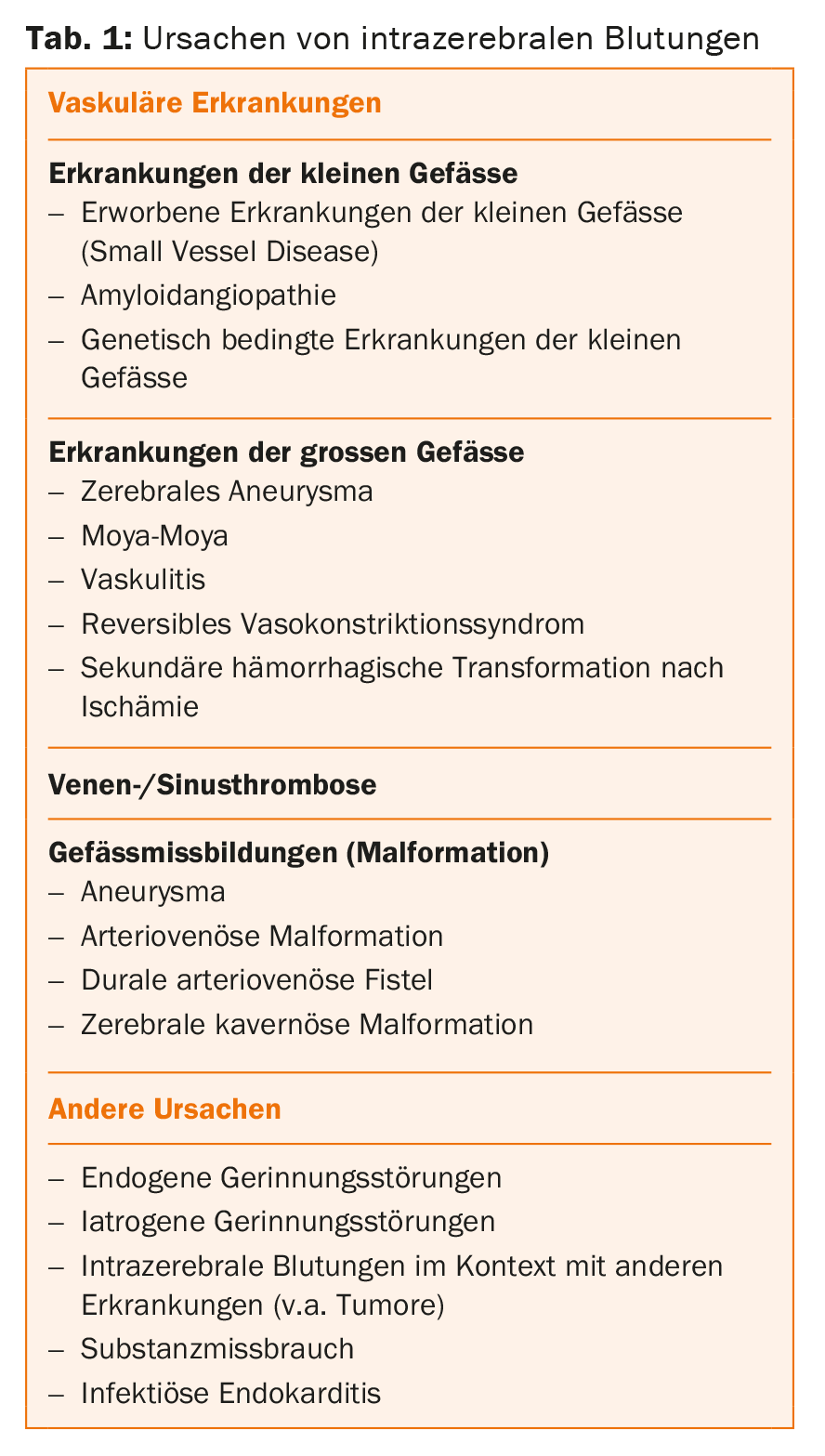
While in older patients with hypertensive ICB the “typical” bleeding is caused by ruptures in the smallest arteries (<0.5 mm), younger patients are more often affected by larger bulges of the vessel wall (aneurysm) or congenital malformations of the vessels (arteriovenous malformations). The latter can be identified noninvasively by CT angiography (CTA) or magnetic resonance imaging (MRI) and, if needed, at the highest resolution by digital subtraction angiography (DSA). In atypical bleeding, especially in young patients, it is important to determine the cause of the bleeding (Table 1).
Spectrum of therapeutic options
Although a large proportion of ICB can be treated conservatively, surgical treatment ranks highly among the wide range of therapeutic options. The constant availability of all therapeutic options in one center is a basic prerequisite for the success of the therapy chain. This chain of therapy consists of emergency first aid, imaging, control of hemostasis, blood pressure adjustment, antiepileptic therapy if necessary, and initiation of internal medicine management to limit primary damage and prophylaxis of secondary damage. For details, see the current American Heart Association guidelines [1]. Possible interventional or surgical measures must be embedded in a functioning overall neurointensive care concept that requires constant availability. In the late stage of acute care, medical management to prevent bleeding recurrence and neurorehabilitation begin.
Surgical therapy subsets include intracranial pressure (ICP) control, hydrocephalus and intraventricular hemorrhage management, and surgical hematoma evacuation.
Control of intracranial pressure
ICP measurement should be used when the Glasgow Coma Scale (GCS) score falls below 9, transtentorial herniation signs are evident, or hydrocephalus is present. Technically suitable is the possibility to insert intraparenchymatous or intraventricular pressure measurement by minitrepanation. In the presence of hydrocephalus, the intraventricular catheter can be used to measure intracranial pressure and simultaneously drain CSF in a controlled manner, thereby reducing intracranial pressure. In any case, monitoring of intracranial pressure is recommended in patients with clouding of consciousness [2].
The possibility of ICP measurement allows intensive care measures to be monitored in a more targeted manner, including maintaining an upper limit of ICP at 20 mmHg and optimizing cerebral perfusion pressure (CPP = mean arterial blood pressure – ICP) roughly between 50-70 mmHg. Corticosteroids should not be used to lower intracranial pressure because the side effects outweigh any potential benefit [3].
Intraventricular hemorrhage
Ventricular system hemorrhage occurs in approximately 45% of patients with spontaneous ICB and is independently associated with worse outcome. The mortality rate increases up to 51%, whereas ICB without intraventricular components have a mortality rate of approximately 20% [4]. Nevertheless, the insertion of an external ventricular drainage in unconscious patients with hydrocephalus is recommended [5].
In recent years, intraventricular lysis therapy with rtPA and endoscopic hematoma evacuation have been technically advanced as minimally invasive and tissue-sparing treatment options. Although evidence reviews today have not yet resulted in a clear recommendation for lysis therapy or endoscopic clearance, consideration should be given to the use of these treatment modalities in selected patients who can clearly benefit from these treatments [1,5]. Due to the minimally invasive approach to hemorrhage, even in the case of deep hematomas, the parenchymal damage is very small when the access route is exposed and, in contrast to actual ICB, usually has no subsequent functional relevance. Overall, the complication rate is low and effective hematoma evacuation can be achieved endoscopically even without lysis therapy (Fig. 2).
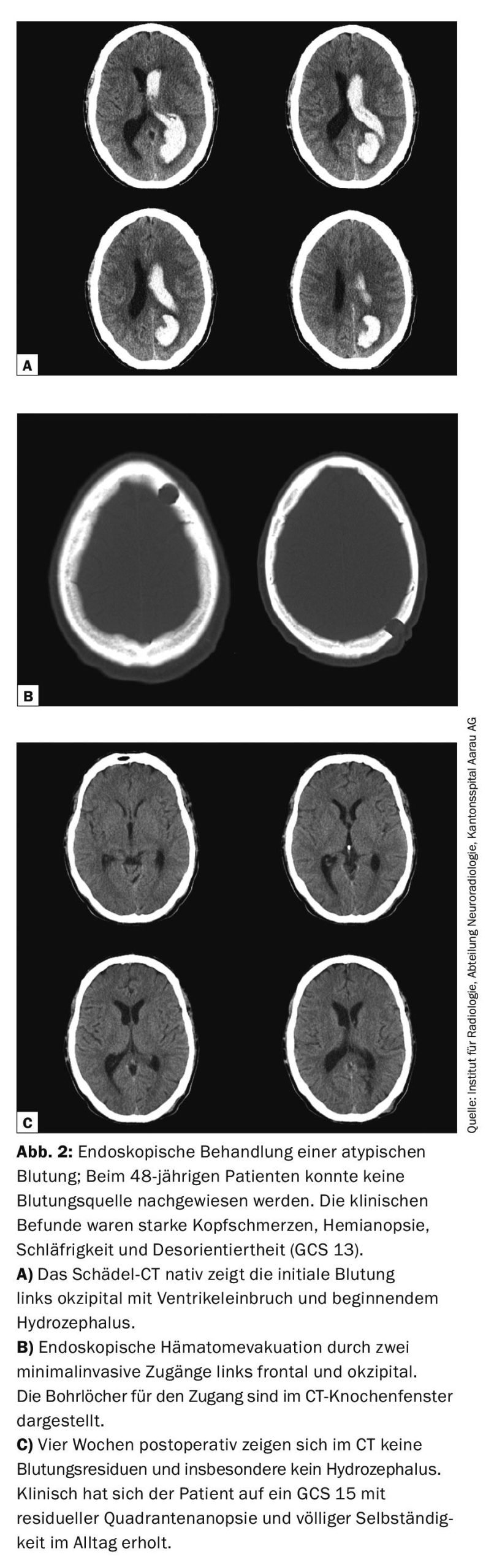
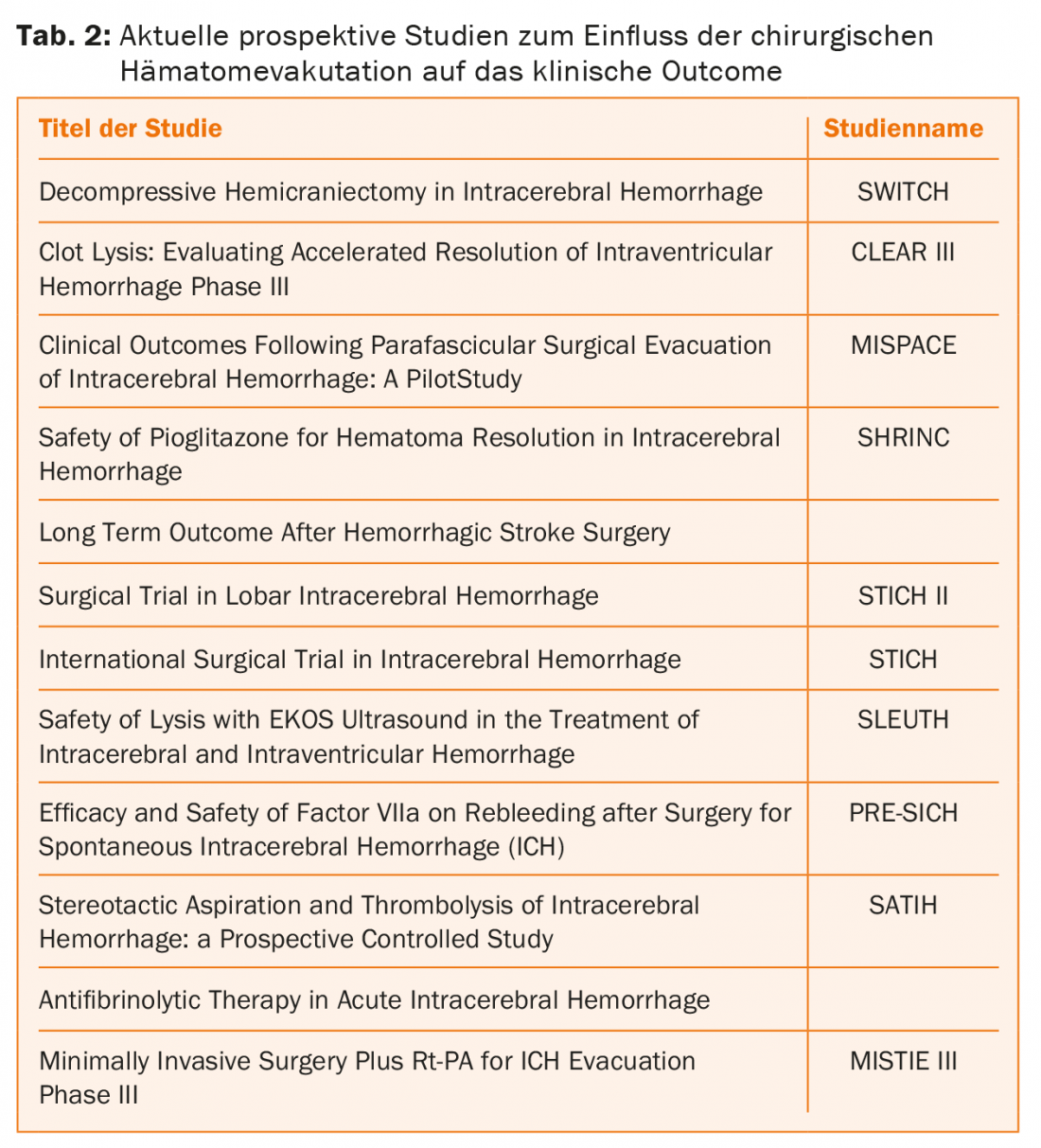
Basaldella et al. were able to show that the reliance on permanent ventriculoperitoneal (VP) drainage after endoscopic hematoma evacuation was significantly less (17%) than with external ventricular drainage or lumbar drainage alone (50%) [6]. Currently, promising prospective randomized trials are ongoing that will provide new insights in this field through their clinically relevant design (Table 2).
Open surgical hematoma evacuation
Even after the large international multicenter randomized trials (STICH I and STICH II), there was no clear class 1 and level A evidence to date favoring early surgical hematoma evacuation (within 24 hours) over conservative therapy [7,8]. However, subgroup analyses of these studies have demonstrated a benefit of early surgical hematoma evacuation in selected patient groups for the following situations:
- Patients with symptomatic cerebellar hemorrhage clearly benefit from early hematoma evacuation. In the presence of hydrocephalus, external ventricular drainage should be inserted despite decompression of the posterior fossa. However, ventricular drainage alone without evacuation of the cerebellar hematoma should be avoided.
- Surgical decompression and hematoma evacuation for cerebral hemorrhage should be considered as a life-sustaining measure if large hematomas with midline displacement and uncontrollable intracranial pressures are involved.
- Other pathophysiologic aspects of hematoma evacuation to consider in atypical near-surface hemorrhage include preservation of the perifocal penumbra, optimized cerebral perfusion pressure by lowering ICP, and reduction of the toxic effect of blood degradation products on healthy brain parenchyma.
Regarding the timing of surgical intervention, in the STICH II trial, subgroup analysis showed an advantage in patients who underwent surgery within 21 hours. In contrast, intervening too quickly (<4 hours) before internal medicine stabilization is associated with an increased risk of rebleeding [9].
For hypertensive, “typical” basal ganglia hemorrhages in elderly patients, conservative therapy remains the method of choice in principle. However, also in this regard, the results of currently ongoing studies for this particular subgroup remain to be seen (Table 2).
Prophylaxis of intracerebral hemorrhage.
Primary health care does its part to prevent ICB. First and foremost, this includes optimal blood pressure control and restrictive indications for anticoagulation. Furthermore, with an increasing number of CT and MRI performed, more and more aneurysms and vascular malformations are being detected incidentally. Dealing with these diseases has become a permanent feature of the specialist centers: In interdisciplinary case discussions, the risk of the natural course is weighed against the risk of treatment for each patient individually. If the risk of possible surgery or intervention outweighs the long-term risk of bleeding, the decision is more likely to be made for an observational approach. On the other hand, if certain risk factors are present, such as a very large aneurysm or a history of ICB in the family, vascular disease care is more likely to be advised. The treatment of aneurysms, arteriovenous malformations or arteriovenous dural fistulas, cavernomas, tumors, and traumatic lesions must be studied and discussed separately accordingly.
Conclusion
Therapy of ICB is a clinical challenge associated with difficult decisions to make. The chances of a positive outcome cannot be optimized based on evidence studies alone. It is important that we not only think and act “evidence based”, but also design the therapy “patient” and “facility based” in order to provide the patient with the optimal therapy with the best possible outcome. The individual clinical situation of the patient, including his or her will or wishes, must be taken into account. the will of the relatives as well as the possibilities of the treating institution are just as important as the correct use of evidence.
Literature:
- Hemphill JC, et al: American Heart Association Stroke Council. Council on Cardiovascular and Stroke Nursing. Council on Clinical Cardiology. Guidelines for the Management of Spontaneous Intracerebral Hemorrhage: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2015 Jul; 46(7): 2032-2060.
- Ko SB, et al: Multimodality monitoring for cerebral perfusion pressure optimization in comatose patients with intracerebral hemorrhage. Stroke 2011; 42(11): 3087-3092.
- Poungvarin N, et al: Effects of dexamethasone in primary supratentorial intracerebral hemorrhage. N Engl J Med 1987; 316(20): 1229-1233.
- Gaberel T, et al: Management of non-traumatic intraventricular hemorrhage. Neurosurg Rev 2012; 35(4): 485-494.
- Naff N, et al: Low-dose recombinant tissue-type plasminogen activator enhances clot resolution in brain hemorrhage: the intraventricular hemorrhage thrombolysis trial. Stroke 2011; 42(11): 3009-3016.
- Basaldella L, et al: External ventricular drainage alone versus endoscopic surgery for severe intraventricular hemorrhage: a comparative retrospective analysis on outcome and shunt dependency. Neurosurg Focus 2012; 32(4): E4.
- Mendelow AD, et al, STICH investigators: Early surgery versus initial conservative treatment in patients with spontaneous supratentorial intracerebral haematomas in the International Surgical Trial in Intracerebral Haemorrhage (STICH): a randomised trial. Lancet 2005; 365(9457): 387-397.
- Mendelow AD, et al: Early surgery versus initial conservative treatment in patients with spontaneous supratentorial lobar intracerebral haematomas (STICH II): a randomised trial. Lancet 2013; 382(9890): 397-408.
- Morgenstern LB, et al: Rebleeding leads to poor outcome in ultra-early craniotomy for intracerebral hemorrhage. Neurology 2001; 56(10): 1294-1299.
InFo NEUROLOGY & PSYCHIATRY 2015; 13(6): 8-13.