Targeted systemic therapies have become increasingly important in both anaplastic and advanced radioiodine-refractory differentiated thyroid carcinoma. These have proven to be effective options over the past several years in the presence of RET, TRK, and BRAF alterations. Only recently, the TRK inhibitors larotrectinib and entrectinib as well as the RET inhibitors selpercatinib and pralsetinib were approved in Switzerland.
At the latest with the approval of specific RET (Rearranged During Transfection) and TRK (Tropomyosin Receptor Kinase) inhibitors, personalized cancer therapy has also arrived in the treatment of thyroid cancer. Thus, advanced radioiodine-refractory tumors are increasingly successfully treated with targeted substances. On the one hand, these have proven to be an effective therapeutic option in the presence of RET, TRK and BRAF alterations. On the other hand, they may serve to induce redifferentiation of the tumor – and thus renewed vulnerability to radioiodine. In addition to targeted agents, immunotherapeutics are increasingly used, particularly in anaplastic thyroid carcinoma. With these new developments, the importance of molecular genetic analysis in thyroid cancer has increased significantly in the last two years. Current research focuses not only on the further development of first-line treatment, but also on the evaluation of therapeutic options for later lines of treatment, which have been little studied to date.
Radioiodine-refractory differentiated thyroid carcinoma: who is who?
Differentiated tumors, especially papillary tumors, account for approximately 85% of the 890 newly diagnosed thyroid carcinomas in Switzerland each year [1]. In 15% of cases, the disease proves to be recurrent or metastatic [2]. Often the tumor then no longer responds to systemic therapy with radioiodine and is thus radioiodine-refractory. This leads to a marked worsening of prognosis with a 10-year survival rate of about 10%, compared with over 50% for radioiodine sensitivity [3]. Thanks to new therapeutic approaches in advanced radioiodine-refractory tumors, the outlook may now improve for those affected whose disease can no longer be treated with radioiodine. Until recently, the multikinase inhibitors sorafenib and lenvatinib were the only drug options in such cases. They are used in symptomatic patients or tumors that are progressive according to RECIST (Response Evaluation Criteria in Solid Tumors) and for which no surgical or radiotherapeutic treatment can be considered (ESMO Guidelines). The response rate (ORR) ranges from 12% to 65%, and the median progression-free survival (PFS) ranges from 10 to 30 months – with not insignificant toxicity [4,5]. In particular, cutaneous side effects, hypertension, and diarrhea often require dose reductions that limit therapeutic potential. This is due to a considerable extent to the lack of specificity of the substances. As multikinase inhibitors that block multiple tyrosine kinases, sorafenib and lenvatinib exhibit some off-target activity with corresponding consequences for compatibility.
Gene fusions and mutations as novel therapeutic targets.
In the course of the last two years, the more specific TRK inhibitors larotrectinib and entrectinib were approved in Switzerland in the presence of an NTRK (neurotrophic tyrosine receptor kinase) gene fusion as the first substances ever to be tumor-agnostic – i.e. independent of tumor entity. In addition, the selective RET inhibitors selpercatinib and pralsetinib have been allowed to be used in differentiated thyroid carcinoma with RET fusion since 2021 [6]. These developments have significantly changed the treatment of advanced radioiodine-refractory thyroid carcinoma. Thus, NTRK and RET fusions should be specifically sought today, as emphasized by Prof. Christine Spitzweg, MD, Director of the Interdisciplinary Thyroid Center at LMU Klinikum München, at the last annual meeting of the DGHO, OeGHO, SSMO, and SGH/SSH [7]. If a corresponding fusion is present, effective treatment options exist with the appropriate inhibitors with a significantly improved toxicity profile compared to multikinase inhibitors. According to the world-renowned expert, this also raises the question of whether RET and TRK inhibitors should be used in the first line of treatment, i.e. before lenvatinib or sorafenib. According to EMA (European Medicines Agency) approval, this is currently not permitted in the EU, but is in Switzerland [6,8]. Numerous data presented at recent congresses support the benefit of TRK and RET inhibitors in advanced radioiodine-refractory differentiated thyroid carcinoma, although phase III trials are lacking to date [7, 9-12]. In addition to use for disease control in advanced cases, single case reports also exist in which selective RET inhibitors have been successfully used to reinduce radioiodine storage [7,13,14]. Such reinduction of radioiodine uptake can also be achieved by the use of BRAF and MEK inhibitors. The basis for the storage of iodine and thus the efficacy of radioiodine therapy is the adequate expression of the sodium iodide transporter. Here, the BRAF-MEK signaling pathway plays an important role. If this is overactive, such as in the presence of a BRAFV600E mutation, there is reduced expression of the transporter and thus a reduction in radioiodine uptake. The net result is a loss of radioiodine sensitivity [7,15,16]. Administration of BRAF and/or MEK inhibitors can restore this in some cases. For example, the BRAF inhibitor dabrafenib was shown in a pilot study to be effective in reinducing expression of the sodium iodide transporter for radioiodine therapy [7,17]. On this basis, dabrafenib is already being used off-label for redifferentiation at some centers, but official approval has not yet been granted [7]. Further studies are currently investigating the combined administration of dabrafenib and the MEK inhibitor trametinib [7,18,19]. The aim of the combined therapy is, in addition to a higher effectiveness, the suppression of escape mechanisms.
Also, in more advanced disease stages, when redifferentiation can no longer be achieved, BRAF and MEK inhibitors represent a new therapeutic option in BRAFV600E mutated tumors [7]. Thus, the response rate with dabrafenib monotherapy as well as with combination therapy with dabrafenib plus trametinib after 1-3 prior TKI treatments is approximately 50% [20]. The median PFS in the corresponding phase II trial was 11.4 months with dabrafenib and 15.1 months with the combination treatment. Although combination treatment was not clearly superior to monotherapy, those patients whose disease progressed on monotherapy with dabrafenib benefited from additional trametinib administration [7]. Unfortunately, neither BRAF nor MEK inhibitors have yet been approved in thyroid carcinoma [6]. Therefore, if a BRAFV600E mutation is present, an individual curative trial must be requested. If redifferentiation and subsequent radioiodine therapy is sought, the timing of therapy is an important factor to consider, according to Prof. Spitzweg. In this case, treatment should begin earlier to create the best possible conditions for radioiodine therapy. A clear consensus regarding the optimal timing for treatment does not exist, however, a redifferentiation trial should be considered in all patients with BRAFV600E mutation [7].
Alternative active ingredient cabozantinib
In addition to targeted TRK, RET, BRAF, and MEK inhibitors, the multikinase inhibitor cabozantinib is currently being evaluated for use in radioiodine-refractory differentiated thyroid carcinoma [7]. In addition to MET and RET, this blocks VEGFR2 (vascular endothelial growth factor receptor 2) in particular [6]. Second- and third-line use after lenvatinib and/or sorafenib has so far shown promise with a median PFS of 12.7 months and a median overall survival (OS) of 34.7 months across all subgroups [7,21]. Currently, the phase III COSMIC-311 trial is ongoing, which in initial analyses also shows a significant PFS benefit of cabozantinib in the second and third lines of treatment compared to placebo (HR 0.22, 96% CI 0.13 – 0.36, p<0.0001) [7,22–24]. As with other multikinase inhibitors, suboptimal tolerability is a minus point of therapy. Nevertheless, cabozantinib could be an effective option for the second- and third-line therapy that has been lacking to date. The substance has been approved in this indication in the USA since September 2021; EMA and Swissmedic approvals are pending [6,7,8].
In summary, in the presence of an appropriate genetic alteration, first-line therapy with selective TRK and RET inhibitors for advanced radioiodine-refractory differentiated thyroid carcinoma has gained acceptance in the last two years. In BRAFV600E mutation, an individual curative attempt and in less advanced cases especially a redifferentiation attempt using BRAF and possibly MEK inhibitor should be considered [7]. Molecular genetic analysis has become significantly more important with the advent of increasingly specific targets. For those patients in whom no driver can be identified, treatment with lenvatinib or sorafenib remains the first-line therapy. In second-line therapy, cabozantinib could soon receive approval. Checkpoint inhibitors such as pembrolizumab are also being investigated in this indication [7].
Developments also in anaplastic thyroid carcinoma
Anaplastic thyroid carcinomas also do not take up iodine – and thus therapeutic radioiodine – due to their dedifferentiation. They account for only 1-3% of all thyroid cancers, but are responsible for half of disease-specific deaths [25]. Classically, therapy consists of surgical resection as well as adjuvant radiochemotherapy. The prognosis is dismal with a median survival of about four to five months, thus there is a high clinical need to develop new therapeutic options [25]. However, this endeavor poses some challenges, not least due to de-differentiation and the accompanying changes in the mutation spectrum. Often, molecular genetic analysis reveals more than 100 different somatic mutations. Particularly common are p53, BRAF, RAS, and β-catenin alterations, which contribute to aggressive tumor growth [25].
The only approved systemic therapies currently available are chemotherapy using doxorubicin and treatment with RET and TRK inhibitors in the presence of appropriate fusion. In addition, chemotherapy with carboplatin/taxol is used [25]. However, with response rates of approximately 25% and median PFS times of 3.4 months with doxorubicin and 4.5 months with carboplatin/taxol, the efficacy of both chemotherapy regimens leaves much to be desired [25]. While doxorubicin or taxol monotherapy is more likely to be used in older, less fit patients, treatment with carboplatin/taxol is preferred in fitter affected individuals because of its marginally better efficacy [25]. Newer treatment approaches include the multikinase inhibitor lenvatinib in combination with checkpoint inhibitors and BRAF inhibitors – so far without approval [25].
About a quarter of patients with anaplastic thyroid carcinoma have a BRAFV600E mutation, which could serve as a new therapeutic target in the future. For example, in a phase II trial, combination therapy with dabrafenib plus trametinib showed partial remission in two-thirds of the 16 patients included [25,26]. The ORR was 69%, and the 1-year PFS rate was 79%. This represents a significant improvement over previous chemotherapeutic options. BRAF inhibitors may also have value in the neoadjuvant setting and thus represent a treatment option for primarily unresectable tumors [25,27].
Unfortunately, however, in most cases no vulnerable driver can be found. As in differentiated radioiodine-resistant thyroid carcinoma, there is then the possibility of using multikinase inhibitors. Because these tumors typically have a hightumor mutational burden(TMB ) and high PD-L1 expression, there has been increasing focus in recent years on combination therapy consisting of the multikinase inhibitor lenvatinib and the checkpoint inhibitor pembrolizumab [25]. In an initial study, six of eight patients showed a partial response, and only one patient experienced progression on therapy [25,28]. After 16 months, a complete remission was observed in half of the patients. Based on these data, the German-centered ATLEP trials were initiated, which were presented by Prof. Christine Dierks, MD, senior physician at the University Hospital Halle, at the annual meeting of the DGHO 2021 [25]. The ATLEP trials are investigating combination treatment of lenvatinib and pembrolizumab in anaplastic and poorly differentiated thyroid carcinoma. Initial phase II results from 26 patients showed an ORR of 38.5% at three months, and within the first two years, two-thirds of patients responded to treatment. The median PFS was 10 months, and the median OS was 11 months [25].
RET inhibitors in focus
RET inhibitors have emerged over the past several years as a new targeted therapy option. So far, the two active ingredients selpercatinib (LOXO-292) and pralsetinib (BLU-667) have been approved in Switzerland in 2021. The two compounds have been developed in parallel and have completed initial Phase I/II trials (ARROW, LIBRETTO-001) [10,11,34]. On the one hand, they may be used in medullary thyroid carcinoma with RET mutation in second line therapy after vandetanib, and on the other hand, in advanced RET fusion-positive differentiated thyroid carcinoma [6].
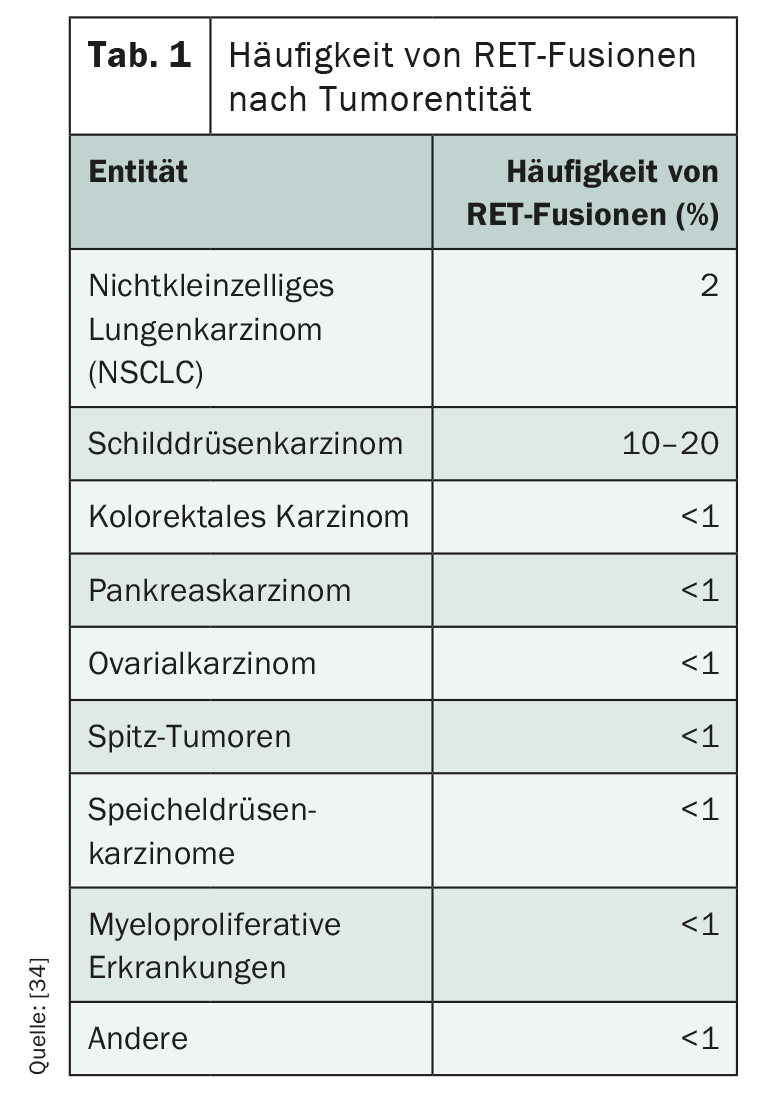
RET alterations play an important role as drivers especially in metastatic thyroid and bronchial carcinomas, but also occur in other entities (Table 1) . While 1-2% of non-small cell lung carcinomas (NSCLC) have such a genetic alteration, RET fusion can be detected in 5-10% of papillary thyroid carcinomas [29]. Children and young adults are particularly frequently affected, and ionizing radiation is a risk factor [30,31]. Unlike papillary thyroid carcinoma, in medullary subtype a point mutation is often crucial for ligand-independent activation of RET receptor kinase [32]. While hereditary multiple endocrine neoplasms (MEN) and familial medullary thyroid carcinoma always have RET germline mutations, somatic RET mutations occur in approximately 60% of sporadic medullary thyroid carcinomas [34]. In most cases, activating RET alterations exclude other driver mutations [34].
In medullary as well as in papillary and follicular thyroid carcinoma, a new therapeutic option now exists in the form of the two agents selpercatinib and pralsetinib, which, based on current data, is not only effective but also better tolerated than the multikinase inhibitors used to date due to their higher selectivity.
In addition, the two new drugs can circumvent an important resistance mechanism that often interferes with multikinase inhibitor therapy [34]. Thus, selpercatinib and pralsetinib bind to RET kinase in a new method. Results from phase III and first-line trials are expected later this year and may lead to the use of pralsetinib and selpercatinib also in the EU as first-line therapy after radioiodine treatment failure [34]. This is already the case in Switzerland [6]. If resistance to either selpercatinib or pralsetinib develops, switching to the other agent is currently not considered promising, as resistance to both agents appears to be characterized by similar mutations [33].
Testing for attackable RET alterations is now mandatory, at least in metastatic thyroid and bronchial carcinomas. NGS (Next Generation Sequencing) is considered the gold standard[34]. In case of lack of availability, RET fusions can be investigated by FISH (fluorescence in situ hybridization) and RET point mutations by PCR (polymerase chain reaction) . However, a positive result from FISH or PCR must be validated by NGS [34]. Currently, liquid biopsy is not a full substitute for fine tissue examination [34]. Not every RET point mutation found in NGS is necessarily predictive of response to targeted therapy. Especially if the tumor has many mutations in different genes and it is not a bronchial or thyroid carcinoma, consultation with experts is advisable before considering RET as a therapeutic target [34].
Literature:
- Krebsliga Schweiz: Cancer in Switzerland: important figures. Status December 2020. www.krebsliga.ch/fileadmin/downloads/sheets/zahlen-krebs-in-der-schweiz.pdf.
- Gild ML, et al: Clinical guidance for radioiodine refractory differentiated thyroid cancer. Clin Endocrinol (Oxf). 2018; 88(4): 529-537.
- Durante C, et al: Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: benefits and limits of radioiodine therapy. J Clin Endocrinol Metab. 2006; 91(8): 2892-2899.
- Schlumberger M, et al: Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N Engl J Med. 2015; 372(7): 621-630.
- Brose MS, et al: Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 3 trial. Lancet. 2014; 384(9940): 319-328.
- Medicinal product information of Swissmedic. www.swissmedicinfo.ch (last accessed 10/22/2021).
- Spitzweg C: Molecularly targeted therapy and novel therapeutic approaches in radioiodine-refractory differentiated thyroid carcinoma. Annual Meeting of the German, Austrian and Swiss Societies of Hematology and Medical Oncology; Berlin, Oct 04, 2021.
- EMA Drug Information. www.ema.europa.eu/en/medicines/human (last accessed 10/22/2021).
- Cabanillas ME, et al: Larotrectinib treatment of advanced TRK fusion thyroid cancer. ESMO Congress 2020, E-Poster Display, Abstract #1916P.
- Subbiah V, et al: Pralsetinib for patients with advanced or metastatic RET-altered thyroid cancer (ARROW): a multi-cohort, open-label, registrational, phase 1/2 study. Lancet Diabetes Endocrinol. 2021; 9(8): 491-501.
- Wirth LJ, et al: Efficacy of selpercatinib in RET-altered thyroid cancers. N Engl J Med. 2020; 383(9): 825-835.
- Sherman EJ, et al: Selpercatinib efficacy and safety in patients with RET-altered thyroid cancer: A clinical trial update. ASCO Annual Meeting 2021, Poster Session Head and Neck Cancer, Abstract #6073.
- Groussin L, et al: Selpercatinib-Enhanced Radioiodine Uptake in RET-Rearranged Thyroid Cancer. Thyroid. 2021.
- Lee YA, et al: NTRK and RET fusion-directed therapy in pediatric thyroid cancer yields a tumor response and radioiodine uptake. J Clin Invest. 2021; 131(18).
- Cabanillas ME, Ryder M, Jimenez C: Targeted Therapy for Advanced Thyroid Cancer: Kinase Inhibitors and Beyond. Endocr Rev. 2019; 40(6): 1573-1604.
- Spitzweg C, et al: Advanced radioiodine-refractory differentiated thyroid cancer: the sodium iodide symporter and other emerging therapeutic targets. Lancet Diabetes Endocrinol. 2014; 2(10): 830-842.
- Rothenberg SM, et al: Redifferentiation of iodine-refractory BRAF V600E-mutant metastatic papillary thyroid cancer with dabrafenib. Clin Cancer Res. 2015; 21(5): 1028-1035.
- Leboulleux S, et al: MERAIODE: A Redifferentiation Phase II Trial With Trametinib and Dabrafenib Followed by Radioactive Iodine Administration for Metastatic Radioactive Iodine Refractory Differentiated Thyroid Cancer Patients With a BRAFV600E Mutation (NCT 03244956). ENDO Congress 2021.
- Leboulleux S, et al: Results of Combination therapy for Re-differentiation in BRAF and RAS mutated DTC. ITOG Annual Meeting 2021.
- Shah MH, et al: Results of randomized phase II trial of dabrafenib versus dabrafenib plus trametinib in BRAF-mutated papillary thyroid carcinoma. ASCO Annual Meeting 2017, Poster Discussion Session Head and Neck Cancer, Abstract #6022.
- Cabanillas ME, et al: Cabozantinib As Salvage Therapy for Patients With Tyrosine Kinase Inhibitor-Refractory Differentiated Thyroid Cancer: Results of a Multicenter Phase II International Thyroid Oncology Group Trial. J Clin Oncol. 2017; 35(29): 3315-3321.
- Brose MS, et al: Cabozantinib versus placebo in patients with radioiodine (RAI)-refractory differentiated thyroid cancer (DTC) who have progressed after prior VEGFR-targeted therapy: results from the phase 3 COSMIC-311 trial. ETA Annual Meeting 2021, Oral Session 3, Abstract #18.
- Brose MS, et al: Cabozantinib versus placebo in patients with radioiodine-refractory differentiated thyroid cancer who have progressed after prior VEGFR-targeted therapy: results from the phase 3 COSMIC-311 trial. ASCO Annual Meeting 2021, Oral Abstract Session Head and Neck Cancer, Abstract #6001.
- Capdevila J, et al: Cabozantinib versus placebo in patients with radioiodine-refractory differentiated thyroid cancer who have progressed after prior VEGFR-targeted therapy: Updated results from the phase III COSMIC-311 trial and prespecified subgroup analyses by prior therapy. ESMO Congress 2021, Mini oral session – NETs and endocrine tumours, Abstract #LBA67.
- Dierks C: Systems therapy of anaplastic thyroid carcinoma. Annual Meeting of the German, Austrian and Swiss Societies of Hematology and Medical Oncology; Berlin, Oct 04, 2021.
- Subbiah V, et al: Dabrafenib and Trametinib Treatment in Patients With Locally Advanced or Metastatic BRAF V600-Mutant Anaplastic Thyroid Cancer. J Clin Oncol. 2018; 36(1): 7-13.
- Wang JR, et al: Complete Surgical Resection Following Neoadjuvant Dabrafenib Plus Trametinib in BRAF(V600E)-Mutated Anaplastic Thyroid Carcinoma. Thyroid. 2019; 29(8): 1036-1043.
- Dierks C, et al: Combination of Lenvatinib and Pembrolizumab Is an Effective Treatment Option for Anaplastic and Poorly Differentiated Thyroid Carcinoma. Thyroid. 2021; 31(7): 1076-1085.
- Integrated genomic characterization of papillary thyroid carcinoma. Cell. 2014; 159(3): 676-690.
- Vanden Borre P, et al: Pediatric, Adolescent, and Young Adult Thyroid Carcinoma Harbors Frequent and Diverse Targetable Genomic Alterations, Including Kinase Fusions. Oncologist. 2017; 22(3): 255-263.
- Ricarte-Filho JC, et al: Identification of kinase fusion oncogenes in post-Chernobyl radiation-induced thyroid cancers. J Clin Invest. 2013; 123(11): 4935-4944.
- Mulligan LM: RET revisited: expanding the oncogenic portfolio. Nat Rev Cancer. 2014; 14(3): 173-186.
- Solomon BJ, et al: RET Solvent Front Mutations Mediate Acquired Resistance to Selective RET Inhibition in RET-Driven Malignancies. J Thorac Oncol. 2020; 15(4): 541-549.
- Weiler D, Gautschi O: RET-altered carcinomas: new RET inhibitors for the treatment of solid tumors. info@oncology. 2021; 01: 8-11.
InFo ONCOLOGY & HEMATOLOGY 2022; 10(1): 32-35.