Historically, neuropathic pain has been classified based on the underlying etiology. However, given the heterogeneity of pain mechanisms, other classification schemes may be more appropriate. In this case, the individual somatosensory profile may provide some clues to pathophysiological disorders of afferent processing. In addition, patient-reported symptoms may also describe sensory qualities.
Patients with neuropathic pain are heterogeneous in etiology, pathophysiology, and clinical presentation. They exhibit a variety of pain-related sensory symptoms and signs, a so-called sensory profile. Different sensory profiles may indicate different classes of neurobiological mechanisms, so subgroups with different sensory profiles may respond differently to treatment.
Mechanism-related ordering principle based on sensory profiles
Using hypothesis-free statistical methods in the database of three multinational research networks (German Neuropathic Pain Research Network (DFNS), IMI-Europain, and Neuropain), one study investigated the identification of subgroups in a large sample of patients with neuropathic pain. Standardized quantitative sensory testing was performed in 902 (test cohort) and 233 (validation cohort) patients with peripheral neuropathic pain of various etiologies. Cluster analysis was performed to form subgroups based on 13 quantitative sensory test parameters. Three distinct subgroups with characteristic sensory profiles were identified and replicated [2].
Cluster 1 (sensory loss, 42%) was characterized by loss of small and large fiber function and the presence of paradoxical heat sensation (PHS). These patients did not suffer from sensory enhancement, except for mild dynamic mechanical allodynia (DMA) in a few patients. Approximately 52% of patients with polyneuropathies fell into this category, indicating a subsiding degeneration of almost all fiber classes. Interestingly, 43% of patients with painful radiculopathy (RAD) had this sensory pattern, indicating severe degeneration of sensory fibers within the affected nerve root. Paradoxical warmth sensations were most common, suggesting that they are triggered by a loss of afferent input, although ostensibly a positive sensory sign possibly related to a central disinhibition process. The sensory profile is similar to that of a compression nerve block. It probably represents the “deafferentation” or “painful hypoesthesia” subgroups described by others. The spontaneous pain is likely due to ectopic action potentials generated at proximal sites of injured nociceptors, such as in the dorsal root ganglion or in deafferent central nociceptive neurons [2].
Cluster 2 was characterized by relatively preserved sensory functions of large and small fibers combined with heat and cold hyperalgesia and low-intensity DMA. This pattern (thermal hyperalgesia) occurred in 33% of all patients with peripheral neuropathic pain regardless of etiology. The fact that cutaneous sensory function was relatively well preserved in one third of all patients despite documented nerve damage suggests that peripheral neuropathic pain may be associated with effective cutaneous regeneration and sensitized nociceptors. The sensory profile resembles that of a UV-B burn lesion and is likely due to peripheral sensitization. It represents the subsets of “irritable nociceptors” described by others. Sensitized nociceptors are associated with overexpression of channels and receptors leading to pathological spontaneous discharges and a lowered activation threshold for thermal (heat and cold) and mechanical stimuli. The persistent hyperactivity of surviving nociceptors may be responsible for persistent pain and may lead to some central sensitization in the dorsal horn of the spinal cord, so that tactile stimuli transmitted via A fibers may activate central nociceptive neurons. As a result, mechanical stimuli induce enhanced pain perception, i.e., pinprick hyperalgesia and DMA. Since these types of mechanical hyperalgesia occurred in only about 20% of patients, peripheral nociceptor drive obviously does not always induce central sensitization [2].
Cluster 3 (mechanical hyperalgesia, 24%) was characterized by predominant loss of cold- and heat-sensitive small fibers in combination with blunt pressure hyperalgesia, pinprick hyperalgesia, and marked and more frequent DMA. The quality of burning pain was more pronounced in this group than in the other groups, consistent with the findings in Guillain-Barré syndrome, in which burning pain was associated with small fiber deficits, and with the concept of synthetic heat rather than peripheral sensitization to heat. The profile was most common in patients with postherpetic neuralgia (PHN) (47%). It resembles the profile elicited by high-frequency electrical stimulation of the skin capable of inducing long-term spinal potentiation and probably corresponds to the subsets of “neurogenic hyperalgesia” or “central sensitization” described by others. Central sensitization is pronounced for mechanical stimuli but not for thermal stimuli. The dissociation of thermal and mechanical hyperalgesia can be explained by differences in the neuronal signaling of thermal and mechanical pain, which begins with peripheral encoding in different subsets of nociceptors. Persistent pain in this subgroup again indicates spontaneous activity in the nociceptive system, which may have its origin in the peripheral and/or central nervous system [2].
Profile-based treatment – subgroups respond better!
Based on this algorithm, further clinical trials classified patients according to similar clusters and tested for differential drug efficacy in a planned secondary analysis. For example, a randomized, placebo-controlled proof-of-concept study evaluated the safety and efficacy of 28-day administration of ISC 17536, a novel, orally available inhibitor of the widely used pain receptor Transient Receptor Potential Ankyrin 1, which mediates nociceptive signaling in small peripheral nerve fibers, was studied in 138 patients with chronic, painful diabetic peripheral neuropathy, using quantitative sensory testing to characterize the patients’ baseline phenotype. The primary end point was the change in mean 24-hour average pain intensity score based on an 11-item numeric pain intensity rating scale from baseline to the end of treatment. In the study, the primary endpoint was not met in the entire patient population. However, in an exploratory, hypothesis-generating subpopulation of patients with preserved small nerve fiber function defined by quantitative sensory testing, ISC 17536 was found to have a statistically significant and clinically meaningful improvement in pain [3].
Another randomized, double-blind, placebo-controlled, phenotype-stratified trial evaluated the efficacy of oxcarbazepine (1800-2400 mg) and placebo in two 6-week treatment periods. The primary efficacy measure was the change in median pain intensity between baseline and the last week of treatment, measured on an 11-point numeric rating scale, and the primary objective was to compare the effect of oxcarbazepine in patients with and without an irritable nociceptor phenotype, defined by hypersensitivity and preserved small nerve fiber function, as determined by detailed quantitative sensory testing. Ninety-seven patients with peripheral neuropathic pain due to polyneuropathy, surgical or traumatic nerve injury, or postherpetic neuralgia were randomized. The intention-to-treat population included 83 patients: 31 with the irritable and 52 with the nonirritable nociceptor phenotype. In the overall sample, oxcarbazepine relieved pain by 0.7 points (on a numerical rating scale of 0-10; 95% confidence interval [CI] 0.4-1.4) more than placebo (p=0.015), and there was a significant interaction between treatment and phenotype of 0.7 (95% CI 0.01-1.4, p=0.047). The number of patients who required treatment to achieve pain relief greater than 50% was 6.9 (95% CI 4.2-22) in the total sample, 3.9 (95% CI 2.3-12) in the irritable group, and 13 (95% CI 5.3-∞) in the nonirritable nociceptor group. The results show that oxcarbazepine is more effective for the relief of peripheral neuropathic pain in patients with irritable versus nonirritable nociceptor phenotype [4].
Another study used mixed-effects repeated-measures models to assess the efficacy of pregabalin compared with placebo in subgroups with induced pain phenotypes (ie, hyperalgesia or allodynia) using data from a recent multinational randomized clinical trial (n=539) in which phenotypic subgroups were identified through the use of a structured clinical examination. The difference in mean pain score between the active group and the placebo group (i.e., delta) after 15 weeks of treatment was -0.76 (p=0.001) for the subgroup with hyperalgesia, compared with 0.19 (p=0.47) for the subgroup that did not have hyperalgesia. The interaction between treatment and phenotype, which tests whether subgroups respond statistically differently to treatment, was significant (p=0.0067). The delta for the subgroup with allodynia was -0.31 (p=0.22), compared to -0.30 (p=0.22) for the subgroup without allodynia (treatment-phenotype interaction p=0.98). These results suggest that hyperalgesia, but not allodynia, predicts response to pregabalin in patients with chronic posttraumatic neuropathic pain [5].
Subgrouping identifies responders
In these and other neuropathic pain studies, the QST* sensory profile was used to identify predictors of treatment response that can be tentatively assigned to the three clusters. For example, patients with a QST outcome profile resembling cluster 2 (“heat hyperalgesia”) showed greater efficacy in a prospective, randomized, placebo-controlled trial of oxcarbazepine, in a preplanned analysis of a placebo-controlled trial of botulinum toxin, and in a retrospective analysis of a trial of topical capsaicin patches without a placebo arm. A retrospective analysis of a placebo-controlled trial of topical lidocaine showed less efficacy. Patients with a QST outcome profile resembling cluster 1 (“sensory loss”) demonstrated greater efficacy in a retrospective analysis of a placebo-controlled trial of oral opioids. A prospective randomized placebo-controlled trial of oxcarbazepine showed less efficacy. Patients with a QST outcome profile resembling cluster 3 (“mechanical hyperalgesia”) demonstrated greater efficacy in retrospective analyses of placebo-controlled trials of oral pregabalin, topical lidocaine, lamotrigine, or intravenous lidocaine.
* QST=standardized protocol for quantitative sensory testing.
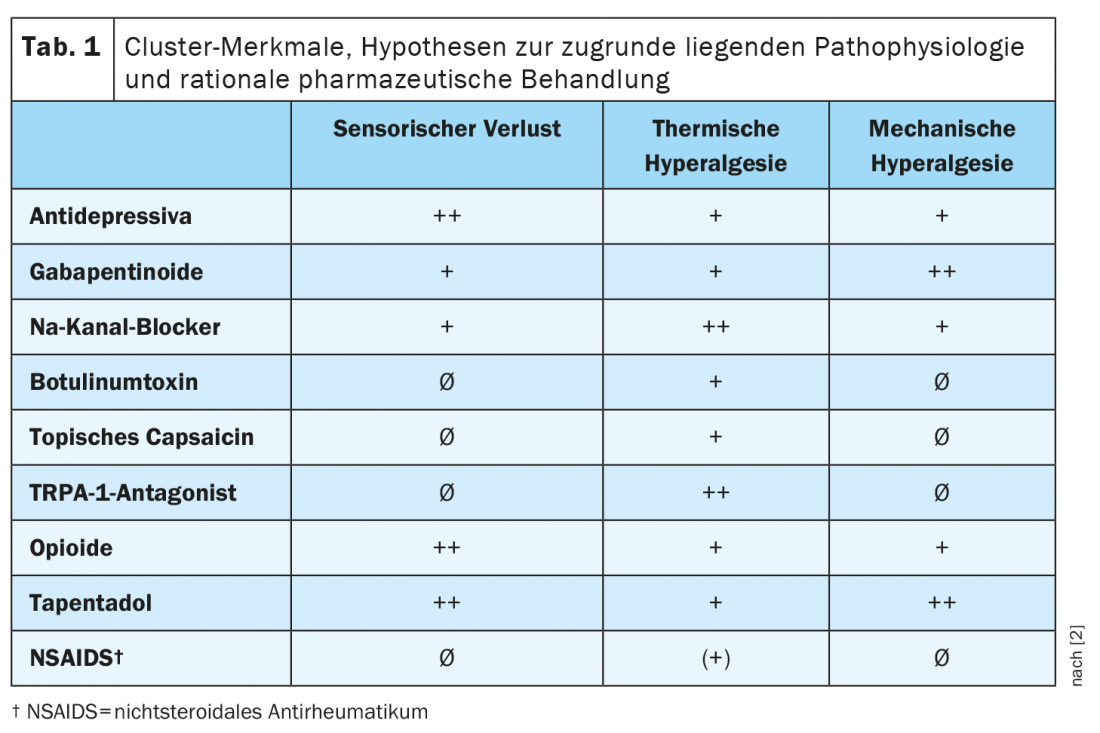
The different pharmacological profiles underline the clinical relevance of the clusters. A prediction of the differential efficacy of the main drugs for neuropathic pain in the different clusters is summarized in Table 1 [2]. Prof. Dr. Ralf Baron, Head, Section Neurological Pain Research and Therapy at the University Hospital Schleswig-Holstein in Kiel, emphasizes at this point that the extent of the difference in response to treatment between the clusters, however, still needs to be demonstrated in future prospective studies [1].
Sensory symptoms captured by patient-reported outcomes (PRO).
In addition to the QST sensory profile, there are also patient-reported symptoms that describe sensory qualities. A patient-reported outcome (PRO) describes information assessed and reported directly by the person about how they feel about their health or treatment, or functioning, without interpretation or modification by others, including clinicians and researchers. For neuropathic or chronic pain patients, there are some validated questionnaires that very specifically ask about different sensory symptoms (Tab. 2) [6–8].
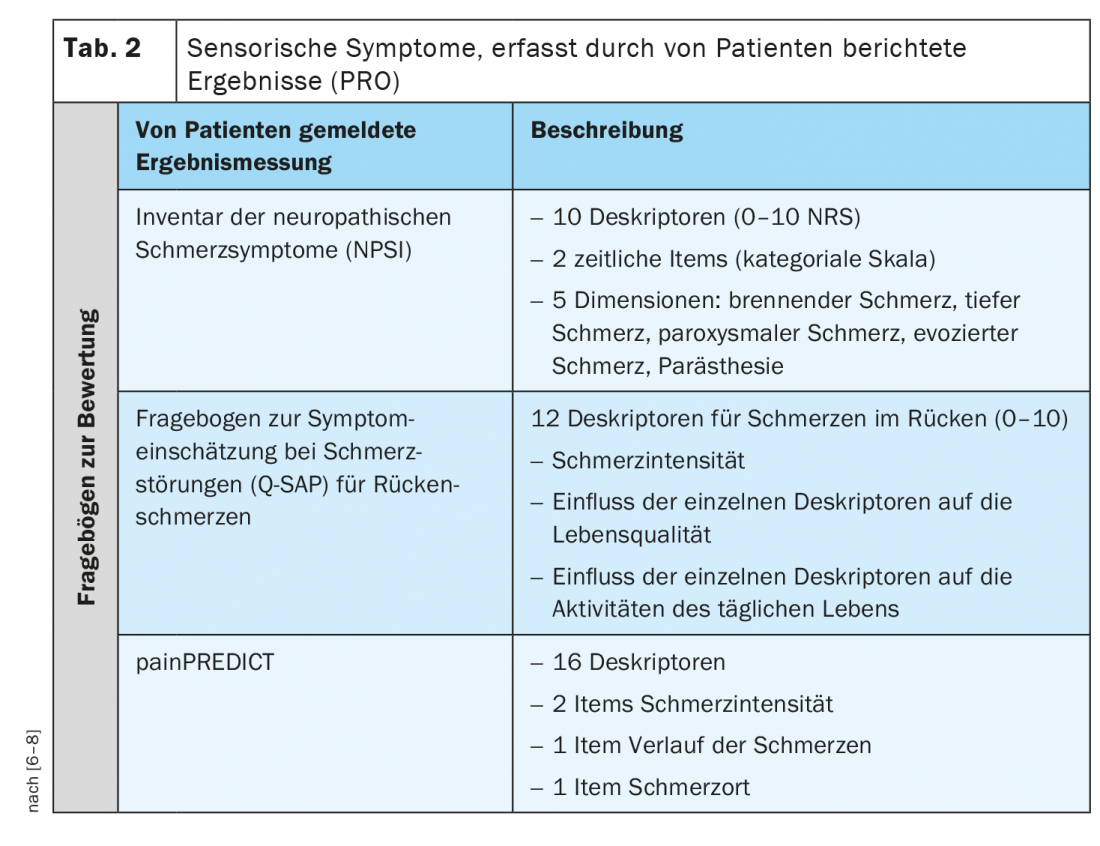
For example, the Neuropathic Pain Symptom Inventory (NPSI) is a self-questionnaire specifically designed to assess the various symptoms of neuropathic pain. The final version of the NPSI includes ten descriptors (plus two temporal items) that allow discrimination and quantification of five distinct, clinically relevant dimensions of neuropathic pain syndromes that are sensitive to treatment. The psychometric properties of the NPSI suggest that it could be used to characterize subgroups of patients with neuropathic pain and to test whether they respond differently to different pharmacological agents or other therapeutic interventions [6].
The Q-SAP is a specific self-questionnaire that assesses the classic nociceptive and neuropathic pain symptoms of patients with chronic low back pain in relation to their local distribution. In addition, the questionnaire captures the intensity of these symptoms and their impact on quality of life and functionality and can be used to evaluate treatment. The self-questionnaire consists of two parts (for back and leg symptoms, if applicable) and was tested on 152 patients with chronic low back pain with and without radiculopathy. Unlike other questionnaires, the Q-SAP Back/Leg assesses not only the intensity of symptoms, but also their impact on the patient’s quality of life and functionality. In addition, this questionnaire interrogates symptoms according to their anatomical distribution [7].
painPREDICT is a questionnaire that uses a wide range of descriptors as indicated by patients, potentially related to neuropathic and nociceptive pain mechanisms, and to investigate sensory symptom patterns. The final questionnaire included 20 items covering the following domains: Pain intensity, location of pain, pain progression, and sensory symptoms. Hybrid clustering of the new questionnaire data revealed three distinct characteristic sensory symptom profiles in patients with neuropathic pain (NeP): “irritable nociceptors,” “deafferentation pain,” and “pain attacks with a nociceptive component.” Although some differences in the distribution of sensory profiles were noted, all profiles were represented in all NeP etiology groups [8].
Take-Home Messages
- There are mechanical subgroups in pain patients
- Some subgroups respond particularly well to opioids
- Assessment tools are:
– QST sensory profiles
– PRO-sensory profiles
Congress: German Pain Congress 2022
Literature:
- Prof. Dr. Ralf Baron: Pain Assessment – The Basis for Individual Therapy. German Pain Congress 2022, Industry Symposium; Oct. 21, 2022.
- Baron R, et al: Peripheral neuropathic pain: a mechanism-related organizing principle based on sensory profiles. Pain 2017; doi: 10.1097/j.pain.0000000000000753.
- Jain M, et al: Randomized, double-blind, placebo-controlled trial of ISC 17536, an oral inhibitor of transient receptor potential ankyrin 1, in patients with painful diabetic peripheral neuropathy: impact of preserved small nerve fiber function. Pain 2022; doi: 10.1097/j.pain.0000000000002470.
- Demant DT, et al: The effect of oxcarbazepine in peripheral neuropathic pain depends on pain phenotype: A randomised, double-blind, placebo-controlled phenotype-stratified study. Pain 2014; doi: https://doi.org/10.1016/j.pain.2014.08.014.
- Gewandter JS, et al: Predicting Treatment Response with Sensory Phenotyping in Post-Traumatic Neuropathic Pain. Pain 2022; doi: 10.1093/pm/pnac045.
- Bouhassira D, et al: Development and validation of the Neuropathic Pain Symptom Inventory. Pain 2004; doi: 10.1016/j.pain.2003.12.024.
- Otto JC, et al: Validation of the Questionnaire for Symptom Assessment in Pain disorders for Back pain patients (Q-SAP). Eur J Pain 2020; doi: 10.1002/ejp.1690.
- Tölle TR, et al: painPREDICT: first interim data from the development of a new patient-reported pain questionnaire to predict treatment response using sensory symptom profiles. Curr Med Res Opin 2019; doi: 10.1080/03007995.2018.1562687.











