The prognosis in relapsed and refractory multiple myeloma (RRMM) remains grim. Now, at the annual meeting of the American Society of Hematology (ASH), data have been presented that raise faint hope for future treatments. Among other things, the focus was on a new target: the BCMA molecule.
With proteasome inhibitors, immunomodulatory drugs (IMiDs) and anti-CD38 antibodies, several agents are already available for the treatment of multiple myeloma. Nevertheless, there are a considerable number of patients whose disease does not respond or responds only temporarily to these substances. In such cases, the need for new strategies is great.
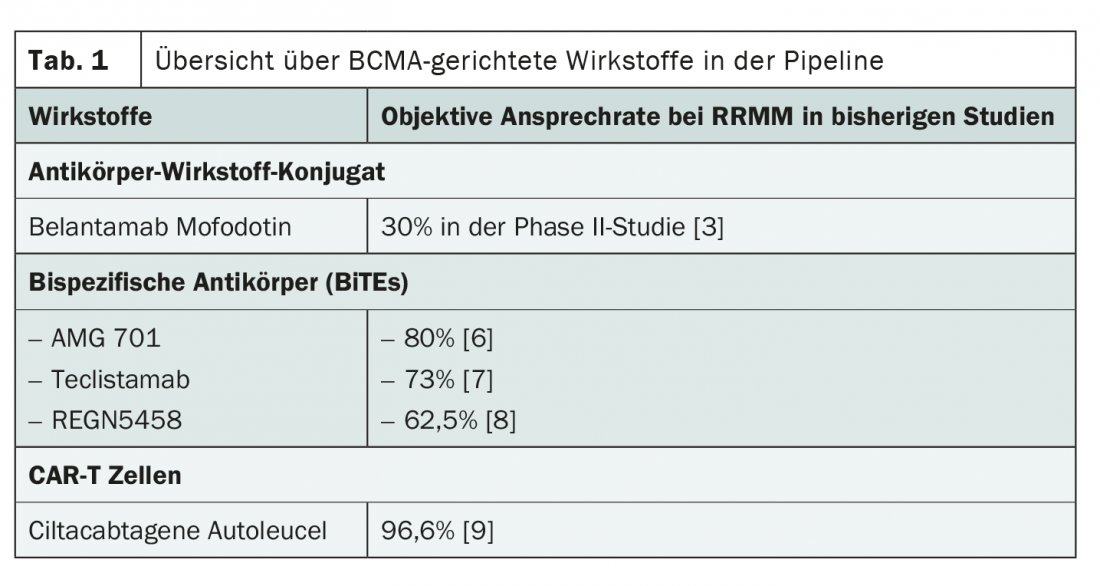
A current approach is the attack of the so-called B-cell maturation antigen (BCMA). This is a surface receptor expressed on differentiated B cells and plasma cells that plays an important role in survival. In the past, it has been shown that there is an above-average amount of BCMA on malignant cells in multiple myeloma. The obvious conclusion to target the molecule was lively discussed at the ASH Annual Meeting in December 2020. In particular, the focus was on three substance classes: the antibody-drug conjugate belantamab mafodotin, bispecific antibodies and CAR-T cells (Table 1).
Antibody-drug conjugate with prolonged survival
Belantamab mofodotin consists of a humanized IgG1 monoclonal antibody to BCMA coupled via a linker to cytotoxic monomethylauristatin F (MMAF), an inhibitor of tubulin polymerization. Upon binding of the antibody to BCMA, the antibody-toxin conjugate is taken up into the cell, resulting in cell death through the release of MMAF. Furthermore, antibody binding recruits endogenous immune cells and blocks BCMA surface receptors, further enhancing the antitumor effect. The drug is administered intravenously every three weeks.
To date, the new compound has been studied in the DREAMM-1 and DREAMM-2 trials, which are currently ongoing [2,3]. Good objective overall response rates of 60% in the phase I trial and just over 30% in the phase II trial were seen in a multiply pretreated patient population. The data suggest that prior therapy with the CD38 antibody daratumumab may have an unfavorable effect on response. In the DREAMM-2 study, the median duration of response was 11 months with a median overall survival of 14.9 months with treatment with belantamab mofodotin. These values could potentially be further increased by the addition of the immunomodulator pomalidomide and dexamethasone, according to recent results presented at the ASH Congress [4].
With overall tolerability, keratinopathy, thrombocytopenia, and anemia were the most common side effects in studies to date. About half of the study participants complained of visual disturbances. In most cases, corneal infestation was self-limiting and ended after about one month, but sometimes led to treatment interruptions. Splitting the dosage and introducing a saturation dose could remedy this situation and reduce the rate of severe keratinopathies [4].
Bispecific antibodies: help for self-help
Receiving more attention than belantamab mofodotin at the ASH Annual Meeting was the potential introduction of bispecific antibodies, known as bispecific T-cell engagers (BiTEs), into myeloma therapy. These bind on the one hand to the BCMA molecule on the tumor cell and on the other hand to the T cell receptor. The immune response thus initiated leads to cell death. Data were presented on three new agents, all of which are promising options.
For the bispecific antibody AMG 420, impressive data with response rates around 70% and a high proportion of complete remissions were already available in advance [5]. Due to its impractical method of application, this substance has now been further developed. With AMG 701, continuous infusion is no longer necessary; instead, the drug is administered intravenously in weekly doses. Initial analyses show consistently high response rates around 80%. Remissions on AMG 701 lasted more than a year in many cases, and as long as 22 months in one case at the time of presentation [6].
Other bispecific antibodies in development include Teclistamab, which is also suitable for subcutaneous application, and REGN5458. Slightly lower response rates of 73% and 62.5%, respectively, were reported for these agents. However, the maximum dose has not been exhausted in either case, which is also reflected in lower toxicities [7,8].
Nearly 100% response to CAR-T cells
CAR-T cells directed against BCMA may also point the way forward in the treatment of relapsed or refractory multiple myeloma. Compared to belantamab mofodotin and bispecific antibodies, the response rates presented at the ASH Annual Meeting were even higher for this form of BCMA-targeted therapy. Initial results from the Phase I CARTITUDE-1 trial, which included heavily pretreated patients, showed an overall response of 96.6%. 67% of the study participants even experienced a complete remission of their disease. 76% were still without recurrence at 12 months and 88.5% of patients were still alive at this time [9].
Compared with bispecific antibody therapy, treatment with CAR-T cells resulted in more hematotoxicity, neurotoxicity, and cytokine release syndromes. However, the rate of infections was comparable in previous studies.
A common goal
Even if the mechanisms of action and thus side effects, efficacies and safety profiles differ, the target remains the same: the BCMA molecule. Or more broadly: To give hope to myeloma patients in previously hopeless situations. Which therapies will ultimately prevail is currently still written in the stars. The potential for early approval exists for several compounds and we hope to see confirmation of the results so far next year. And also in the year after.
Source: 62nd Annual Meeting of the American Society of Hematology (ASH Annual Meeting), December 5-8, 2020, virtual conduct.
Literature:
- Gandhi UH, et al: Outcomes of patients with multiple myeloma refractory to CD38-targeted monoclonal antibody therapy. Leukemia. 2019; 33(9): 2266-2275.
- Trudel S, et al: Targeting B-cell maturation antigen with GSK2857916 antibody-drug conjugate in relapsed or refractory multiple myeloma (BMA117159): a dose escalation and expansion phase 1 trial. Lancet Oncol. 2018; 19(12): 1641-1653.
- Lonial S, et al. Belantamab mafodotin for relapsed or refractory multiple myeloma (DREAMM-2): a two-arm, randomised, open-label, phase 2 study. Lancet Oncol. 2020; 21(2): 207-221.
- Trudel S, et al: Part 1 Results of a Dose Finding Study of Belantamab Mafodotin (GSK2857916) in Combination with Pomalidomide (POM) and Dexamethasone (DEX) for the Treatment of Relapsed/Refractory Multiple Myeloma (RRMM). 62nd ASH Annual Meeting Dec 2020. Abstract #725.
- Topp MS, et al: Anti-B-Cell Maturation Antigen BiTE Molecule AMG 420 Induces Responses in Multiple Myeloma. J Clin Oncol. 2020; 38(8): 775-83.
- Harrison S, et al: A Phase 1 First in Human (FIH) Study of AMG 701, an Anti-B-Cell Maturation Antigen (BCMA) Half-Life Extended (HLE) BiTE ® (bispecific T-cell engager). Molecule, in Relapsed/Refractory (RR) Multiple Myeloma (MM). 62nd ASH Annual Meeting Dec 2020. Abstract #181.
- Garfall A., et al: Updated Phase 1 Results of Teclistamab, a B-Cell Maturation Antigen (BCMA) x CD3 Bispecific Antibody, in Relapsed and/or Refractory Multiple Myeloma (RRMM). 62nd ASH Annual Meeting Dec 2020. abstract #180.
- Madduri D, et al: REGN5458, a BCMA x CD3 Bispecific Monoclonal Antibody, Induces Deep and Durable Responses in Patients with Relapsed/Refractory Multiple Myeloma (RRMM). 62nd ASH Annual Meeting Dec 2020. abstract #291.
- Madduri D, et al: CARTITUDE-1: Phase 1b/2 Study of Ciltacabtagene Autoleucel, a B-Cell Maturation Antigen-Directed Chimeric Antigen Receptor T Cell Therapy, in Relapsed/Refractory Multiple Myeloma. 62nd ASH Annual Meeting Dec 2020. Abstract #177.
InFo ONCOLOGY & HEMATOLOGY 2021; 9(1): 34-35 (published 2/22/21, ahead of print).