Therapy for AML and ALL is on the move. In the future, it will be much more differentiated. Some new options have already been approved, while others are in development or on their way to clinical use. For therapy management, minimal residual disease is increasingly coming to the fore.
The therapeutic landscape of AML and ALL is changing and will become much more differentiated in the future. The following overview focuses on new developments that have already been approved by Swissmedic or by other authorities such as the FDA or EMA, or that could soon be available to patients in Switzerland as part of clinical trials or “early access” programs.
In AML, these include long-established drugs that improve therapeutic options through new formulations, as well as immunotherapeutic options through monoclonal antments against pathogenetically relevant molecular targets (FLT-3, IDH, BCL-2).
In acute lymphoblastic leukemia, next-generation sequencing has identified novel prognostic markers, targets, and subtypes. Therapeutically, monoclonal antibodies, bispecific antibody constructs, and chimeric antigen receptor T cell (CAR-T cell) therapies are in development and some are already on their way to clinical use.
For the management of acute leukemia therapy, assessment and treatment of minimal residual disease are increasingly coming to the fore.
Monoclonal antibodies – gemtuzumab ozogamicin (Mylotarg®) in AML
Gemtuzumab ozogamicin (GO) is a monoclonal antibody against CD33 covalently linked to a very potent cytotoxin (calicheamicin), which exerts its cytotoxic effect after internalization. After GO was approved by the FDA several years ago for the treatment of AML in elderly patients based on surrogate parameters, doubts about efficacy later emerged based on further study data, while unexpected toxicities (liver) were observed, leading to the withdrawal of the drug by the manufacturer. Later, a significant overall survival benefit was demonstrated in a meta-analysis based on individual patient data from five controlled randomized trials (OR 0.90; p=0.01). The overall survival benefits were most pronounced, particularly for patients classified in the “good” prognostic group based on cytogenetic aberrations, with an absolute improvement in overall survival at six years of 20.7% (OR 0.47; p=0.0006). For patients at intermediate risk, the absolute benefit was 5.7% (OR 0.84; p=0.005), whereas for patients in the worst risk category, no
relevant profit was demonstrated by GO [1].
In several academic-initiated studies, dosing and sequence of administration could be further developed, resulting in improvements in toxicity with the same efficacy. Since 2017, GO has again been approved by the FDA and, since a few months ago, by the EMA as a combination therapy with daunorubicin and cytarabine (AraC) for the first-line treatment of AML.
New drug formulation: CPX-351
CPX-351(trade name: Vyxeos®) is a liposomal combination preparation of cytarabine and daunorubicin. This compound was compared to conventional “7+3” induction therapy in a randomized phase III trial of 309 patients aged 60 to 75 years with high-risk AML. This showed significantly higher remission rates (CR/CRi 47.7% vs. 33.3%) and improved overall survival (HR 0.69; p=0.003, median 9.6 vs. 6 months) for CPX-351 compared to conventional induction therapy. The 2-year survival rates were 31% and 12%, respectively [2]. CPX-351 was approved by the FDA for AML therapy in June 2017 and has also recently received a positive assessment from the relevant committee of the EMA, so approval can also be expected here shortly. CPX-351 may improve therapy in elderly patients with high-risk AML.
New oral drugs for AML
Tyrosine kinase inhibitors (TKIs) – FLT3 inhibition: A mutation in the tyrosine kinase FLT3 can be detected in approximately 25% of patients with AML. By adding the oral FLT3 inhibitor midostaurin to conventional chemotherapy, the therapeutic results of standard chemotherapy can be relevantly improved. In the international, placebo-controlled, randomized phase III “RATIFY” trial, midostaurin significantly improved median overall survival versus the placebo arm (74.7 months vs. 25.6, p=0.009) and 4-year survival (51.4% vs. 44.3%; HR 0.78; p=0.009). This benefit was consistently demonstrable in the different subgroups of FLT3 mutations (high or low FLT3 ITD allelratio, FLT3 TKD mutations) as well as with and without censoring for allogeneic hematopoietic stem cell transplantation [3].
Promising clinical activity has been demonstrated for the more selective FLT3 inhibitor gilteritinib in AML relapses with FLT3 mutation [4]. The HOVON/SAKK study group is now preparing a study to be conducted in Switzerland comparing these two FLT3 inhibitors as an adjunct to standard chemotherapy in first-line therapy.
IDH2 inhibition: recurrent isocitrate dehydrogenase 2 (IDH2) mutations can be detected as a pathogenetically relevant target structure in approximately 12% of patients with AML. Enasidenib is an oral inhibitor of mutant IDH2. In a prospective single-arm study, patients with relapsed or refractory AML (ref./ref. AML) and IDH2 mutations showed remarkable efficacy for enasidenib monotherapy, with an overall response rate of 39% and a CR rate of 20% [5].
Enasidenib has been FDA approved since 2017 based on these data. Currently, an international phase III trial is recruiting patients with pre-treated AML with IDH2 mutations to compare therapy with enasidenib versus standard of care. In addition, a phase III HOVON/SAKK trial is underway to evaluate IDH inhibition in first-line therapy in addition to induction therapy.
Combination therapies with BCL-2 inhibitor venetoclax in elderly patients: For elderly patients who do not qualify for intensive induction therapy, low-dose cytarabine or the hypomethylating agents azacitidine and decitabine are available in addition to purely supportive therapy, with overall response rates – depending on the study – around 50% (CR/CRi rates 7-47%) and median survival times ranging from 5 to 24 months. Several prospective single-arm studies tested the combination of the BCL-2 inhibitor venetoclax with low-dose cytarabine or hypomethylating agents. This showed promising remission rates of up to 60% for the different combinations and longer response times in historical comparison with satisfactory tolerance [6–8].
Increasing importance of minimal residual disease (MRD).
A prominently published retrospective analysis of multiple HOVON/SAKK trials demonstrated that the absence of MRD detection (flow cytometric or molecular genetic) after induction therapy was associated with an improvement in recurrence-free survival. The longest relapse-free survival was observed for those patients in whom minimal residual disease was not detectable by either flow cytometry or PCR [9]. From this, the concept is now derived that the indication for an allogeneic blood stem cell transplantation, in addition to the conventional genetic risk classification, should also include the MRD status. This is being investigated in a HOVON/SAKK study, patient recruitment of which has been completed.
Novel prognostic markers and targets in ALL.
In just over 80% of patients with Philadelphia pos. B-ALL (Ph+ ALL), a mutation/deletion of the transcription factor IKZF1 is also found, which is associated with therapy resistance and increased relapses. IKZF2 mutations are a hallmark of deeply hypodiploid B-ALL and IKZF3 alterations are found in most near-haploid ALL.
“Ph-like” or “BCR-ABL1 like” ALL show a comparable gene expression profile to Ph+ ALL, but do not have a classic BCR-ABL mutation. 20-30% of all B-ALL patients can be assigned to this group. They have significantly lower PFS and OS of 20-25% vs. approximately 50-55% [10]. About half of the patients with “Ph-like” ALL have a rearrangement of “cytokine receptor-like factor 2” (CRLF2). In addition, a mutation with activation of Janus kinase is found in approximately 50% of patients with CRLF2 alteration [11]. In patients without CRLF2 rearrangement, fusions with tyrosine kinases were detected.
In addition to “Ph-like” ALL, “early T precursor” ALL (ETP-ALL) was newly included as a separate entity in the WHO 2016 classification, based on its own immunophenotyping and gene expression profile. Approximately 35% of all T-ALL can be assigned to this entity, which may be associated with a somewhat less favorable prognosis.
Activation mutations in the NOTCH1 pathway can be found in over 50% of T-ALL patients and are associated with a better prognosis, whereas PTEN deletions and NRAS mutations are associated with a worse prognosis.
In hypodiploid ALL, two subtypes were identified: a group with near-haploid chromosome set (24-31 chromosomes) with mutations in the tyrosine kinase and RAS pathways in approximately 71% or IKZF3 mutations in approximately 13%, and a group with low hypodiploid ALL (32-39 chromosomes) with alterations in TP53 (91%), IKZF2 (53%), and RB1 (41%).
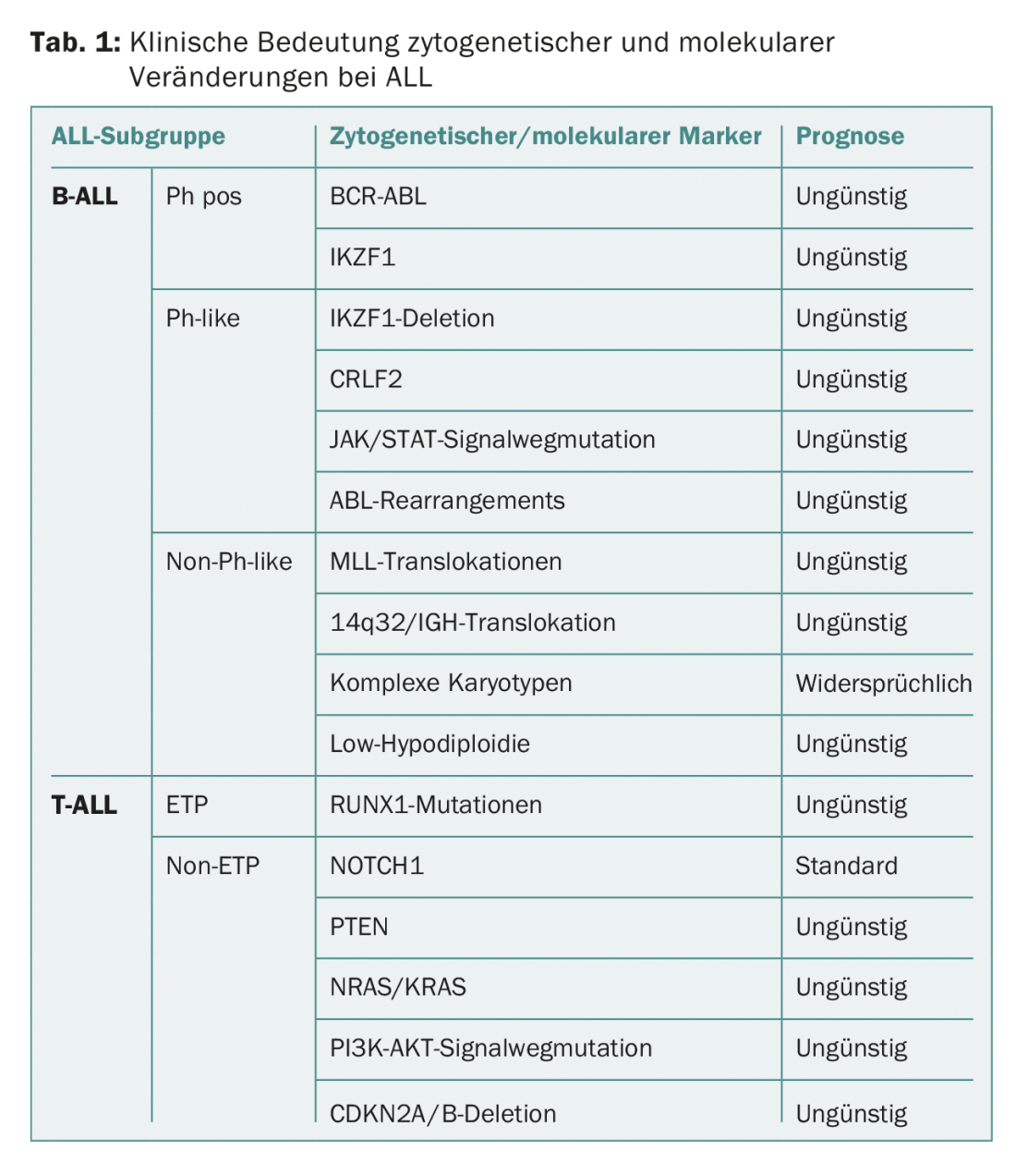
Table 1 provides an overview of the clinical significance of cytogenetic and molecular alterations in ALL.
Minimal residual disease
Earlier detection of lack of response to chemotherapy or recurrence is possible with determination of MRD. In patients with ALL, this is done by PCR methods and detection of clonally rearranged genes encoding immunoglobulin or T-cell receptors, or detection of a leukemia-specific phenotype via multiparameter flow cytometry. A positive event at a frequency of <10-4 is recognized as MRD negativity, or an MRD level of >10-3 is recognized as MRD positive. Achievement of MRD negativity after induction and consolidation therapy is an important prognostic factor that is likely to complement or replace traditional clinical and cytogenetic factors in the future.
Antibody
CD20: 30-40% of B-ALL cases show expression of CD20. The addition of rituximab to standard chemotherapy results in higher and faster MRD negativity after induction and consolidation therapy. Encouragingly, this may result in improved disease-free and overall survival [12,13].
CD22: Since virtually all B-ALL express CD22, this is an ideal target. Inotuzumab ozogamicin (IO) is a conjugate of a CD22 antibody with calicheamicin, which is internalized after binding to the antigen and causes breaks in DNA. Clinical trials have shown a survival benefit with this agent compared with chemotherapy-based therapies in the relapse setting [14]. Currently, IO is being investigated in first-line therapy. Other CD22 antibodies are also being tested in clinical trials.
Blinatumomab: This is a bispecific antibody construct that binds simultaneously to normal CD3+ T cells and CD19+ B-ALL cells, leading to a T cell-mediated cytotoxic response against the B cell. Initial studies with blinatumomab in adults were done with the goal of eliminating minimal residual disease. In a long-term follow-up, twelve of 20 patients remained in CR, with nine patients in this study receiving consolidative allogeneic HSCT. Of note, the nontransplanted patients showed a comparable outcome. In a study of heavily pretreated patients with relapsed B-ALL, blinatumomab was compared with chemotherapy. There were significant differences with CR/CRi of 44% vs. 25% and median overall survival of 7.7 vs. 4.0 months [15].
CD38: Another promising target in T-ALL is CD38, as T-ALL blasts show stably high CD38 expression and normal myeloid and lymphoid cells show only low expression. Daratumumab is a CD38 monoclonal antibody used in multiple myeloma with a good efficacy and safety profile. Xenograft models as well as single case observations showed a convincing effect [16].
CAR-T cells
A new promising option is the use of so-called CAR-T cells. These are patient-derived (autologous) T cells that have been modified ex vivo by genetic engineering to contain a chimeric T cell receptor. Through this, they bind to tumor antigens (e.g. CD 19 on B cells), causing selective elimination of cells carrying this antigen. Currently, only CAR-T cells directed against CD19 are approved by the regulatory authorities (FDA) for the treatment of refractory childhood ALL and refractory B-cell lymphomas. In the corresponding pivotal ALL trials, CR rates were 67-91% with MRD negativity of 60-81% of patients in complete remission. Relevant toxicities (cytokine release syndrome, neurotoxicity) have to be considered during therapy with CD19-CAR-T cells. After therapy of refractory ALL with CD19-targeted CAR-T cells, CD19-negative relapses occur in approximately 20% of patients and are difficult to treat. The value of consolidating allogeneic HSCT after CAR T-cell therapy is controversial.
Summary
After a prolonged period without effective innovations in the therapy of acute leukemias, we are now facing the beginning of a new era with various new therapeutic options, especially immunotherapies, which promise a significant improvement in prognosis and some of which have already been shown in trials. In AML, new substances, so-called “small molecules”, are also being tested in trials or are already in clinical use in some cases. Increasingly clear is the importance of MRD determination during follow-up and, if still present, elimination of residual disease as a prognostic marker.
Take-Home Messages
- The therapeutic landscape of acute myeloid leukemia (AML) is becoming increasingly differentiated. Survival benefits have been demonstrated for a new liposomal formulation of the classic chemotherapy agents, the renaissance of gemtuzumab ozogamicin, and new combinations of tyrosine kinase inhibitors (TKIs) with conventional chemotherapy.
- Molecular analyses and monitoring of minimal residual disease (MRD) are becoming increasingly important and relevant for treatment decisions in AML.
- The use of MRD determinations in therapeutic algorithms in all subtypes of acute lymphoblastic leukemia (ALL) is becoming more routine and may replace most prognostic factors in the future.
- Many new antibody therapies are establishing themselves in relapsed ALL and are also being investigated in clinical trials in first-line therapy. As a new option, CAR-T cell therapy is on the verge of routine use.
Literature:
- Hills RK, et al: Addition of gemtuzumab ozogamicin to induction chemotherapy in adult patients with acute myeloid leukaemia: a meta-analysis of individual patient data from randomised controlled trials. Lancet Oncol 2014; 15(9): 986-996.
- Lancet JE, et al: CPX-351 (cytarabine and daunorubicin) Liposome for Injection Versus Conventional Cytarabine Plus Daunorubicin in Older Patients With Newly Diagnosed Secondary Acute Myeloid Leukemia. J Clin Oncol 2018; 38(26): 2684-2692.
- Stone RM, et al: Midostaurin plus chemotherapy for Acute Myeloid Leukemia with a FLT3 mutation. N Engl J Med 2017; 377(5): 454-464.
- Perl AE, et al: Selective inhibition of FLT3 by gilteritinib in relapsed or refractory acute myeloid leukaemia: a multicentre, first-in-human, open-label, phase 1-2 study. Lancet Oncol 2017; 18(8): 1061-1075.
- Stein EM, et al: Enasidenib in mutant IDH2 relapsed or refractory acute myeloid leukemia. Blood 2017; 130(6): 722-731.
- DiNardo C, et al: Safety and preliminary efficacy of venetoclax with decitabine or azacitidine in elderly patients with previously untreated acute myeloid leukaemia: a non-randomised, open-label, phase 1b study. Lancet Oncol 2018; 19(2): 216-228.
- DiNardo C, et al: Durable Response with Venetoclax in Combination with Decitabine or Azacitidine in Elderly Patients with Acute Myeloid Leukemia. European Hematology Association Congress 2018; Abstract S1563.
- Wei A, et al: Phase 1/2 Study of Venetoclax with Low-Dose Cytarabine in Treatment-Naive, Elderly Patients with Acute Myeloid Leukemia Unfit for Intensive Chemotherapy: 1-Year Outcomes. ASH Annual Meeting and Exposition 2017; Abstract 890.
- Jongen-Lavrencic M, et al: Molecular minimal residual disease in acute myeloid leukemia. N Engl J Med 2018; 378(13): 1189-1199.
- Roberts KG, et al: High frequency and poor outcome of Philadelphia Chromosome-Like Acute Lymphoblastic Leukemia in Adults. J ClinOncol 2017; 35(4): 394-401.
- Herold T, et al: Adults with Philadelphia chromosome-like acute lymphoblastic leukemia frequently have IGH-CRLF2 and JAK2 mutations, persistence of minimal residual disease and poor prognosis. Haematologica 2017; 102(1): 130-138.
- Thomas DA, et al: Chemoimmunotherapy with a modified hyper-CVAD and rituximab regimen improves outcome in de novo Philadelphia chromosome-negative precursor B-lineage acute lymphoblastic leukemia. JCO 2010; 28: 3880.
- Maury S, et al: Addition of Rituximab Improves the Outcome of Adult Patients with CD20-Positive, Ph-Negative, B-Cell Precursor Acute Lymphoblastic Leukemia (BCP-ALL): Results of the Randomized Graall-R 2005 Study. ASH Meeting 2015; 126: 1 .
- Kantarjian HM, et al: Inotuzumab ozogamicin versus standard therapy for acute lymphoblastic leukemia. N Engl J Med 2016; 375(8): 740-753.
- Kantarjian HM, et al: Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia. N Engl J Med 2017 Mar 2; 376(9): 836-847.
- Bride KL, et al: Preclinical efficacy of daratumumab in T-cell acute lymphoblastic leukemia. Blood 2018 Mar 1; 131(9): 995-999.
InFo ONCOLOGY & HEMATOLOGY 2018; 32-35.