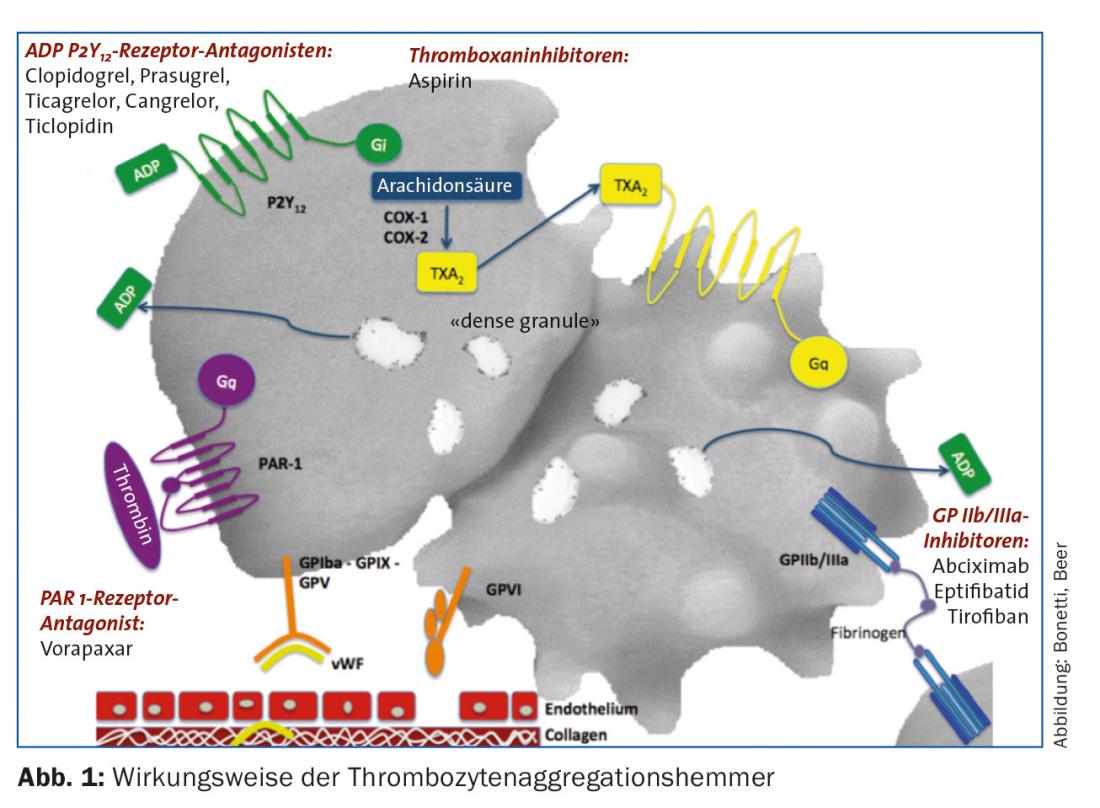
Inhibition of coagulation is an important cornerstone of therapy for acute coronary syndrome. For this purpose, platelet aggregation inhibitors targeting thromboxane synthesis, the ADP receptor, the glycoprotein receptor IIb/IIIa and the thrombin receptor PAR 1 are in clinical use, along with inhibitors of plasmatic coagulation. More potent representatives of individual substances, as well as their combination, promise improved antithrombotic efficacy, but may need to be weighed against bleeding risk on an individual basis.
The path from the first description of angina pectoris by John Warren as “strangling of the chest” in the first issue of the New England Journal of Medicine in 1812 [1] to today’s understanding of coronary artery disease at the genetic and molecular level has been lined with major discoveries, innovations, and outstanding therapeutic advances.
Whereas until 1961 ACS patients had to rest as undisturbed as possible and died in 30% of cases, today they are continuously monitored in coronary care units, which together with the expanded therapeutic intervention options has led to a reduction in hospital mortality to 6-7%.
Therapeutic intervention options include aortocoronary bypass surgery, which celebrated its 50th anniversary in 2014, and percutaneous coronary intervention (PCI), which was pioneered by Andreas Grüntzig in Zurich in 1977. This is now one of the most common interventions ever in modern medicine [2] with annual implantation rates of approximately 500,000 drug-eluting stents (DES), now second generation, in the United States [3]. Given the pathogenetic essential mechanism of local thrombus formation, anticoagulant therapy is of utmost importance. This is composed of inhibition of primary hemostasis and plasmatic coagulation. Therapeutic inhibition of platelet adhesion/activation and aggregation occurs via inhibition of thromboxane synthesis, blockade of the ADP receptor, and inhibition of the GP IIb/IIIa receptor. Inhibition of various factors in the coagulation cascade ensures plasmatic anticoagulation, which in the case of PCI is employed by heparin, low-molecular-weight heparins, and selective inhibitors of specific factors (bivalirudin for thrombin inhibition; fondaparinux for antithrombin-mediated FXa inhibition).
The purpose of this review is to discuss the mechanisms of action and indications for use of currently used antiplatelet agents. For the sake of clarity, anticoagulation in PCI, which is equally important, is not discussed here and can be found elsewhere [4,5].
Platelet Physiology
Platelets are anuclear cells responsible for primary hemostasis via adhesion, aggregation, secretion, and interaction with coagulation factors. Injury to vascular integrity with exposure of the subendothelial matrix results in immediate platelet activation. This is initially mediated by the contact of circulating platelets with collagen, von Willebrand factor, and fibrinogen via glycoprotein receptors VI, Ib, and IIb/IIIa, and is amplified by secretion of, among others, thromboxane and ADP in the sense of a positive feedback loop. The heterodimeric glycoprotein receptor GP IIb/IIIa, which is present in large numbers (approximately 80,000/P) and has a lower affinity for its ligands in quiescent platelets, undergoes a conformational change via intracellular signaling mechanisms that results in exposure of the binding site for ligands such as fibrinogen, vWF, and fibronectin. Platelet aggregation and firm adhesion are mediated by this.
In the setting of acute coronary syndrome, this process can be interrupted therapeutically at the following points (Fig. 1):
- Inhibition of thromboxane synthesis (acetylsalicylic acid [ASS] inhibits cyclooxygenase).
- Inhibition of ADP receptor P2Y12 (irreversible: clopidogrel, prasugrel; reversible: ticagrelor, cangrelor [extremely short acting, suitable for bridging]).
- GP IIb/IIIa receptor inhibition (abciximab, eptifibatide, tirofiban).
- Thrombin receptor inhibition (vorapaxar).
The following is an overview of the individual substances and their mechanisms of action.
Aspirin
Aspirin irreversibly inhibits cyclooxigenase 1 (COX-1) and thus thromboxane A2 synthesis. This effect persists throughout the life span of the platelet (ten days, rule of thumb: 10% “fresh” Tc daily). The efficacy of aspirin has been demonstrated in patients with unstable angina pectoris (AP). Four randomized controlled trials from the pre-PCI era demonstrated a 51% reduction in the rate of myocardial infarction as well as mortality [6–9]. A meta-analysis of these studies demonstrated a 46 percent reduction in the odds ratio for vascular complications in this patient population after two years of aspirin therapy.
Regarding dosing, the CURRENT-OASIS 7 trial, which studied approximately 25,000 patients with ACS, showed no differences in outcome between higher (300-325 mg) and lower dosing (75-100 mg). In fact, the antiaggregatory effects are already saturated at a dose around 30-75 mg at steady-state, whereas gastrointestinal side effects and bleeding rates increase in a dose-dependent manner (even in the lower dose range). Accordingly, in Switzerland a standard dose of 100 mg is recommended in primary and secondary prevention. Primary prevention, on the other hand, is recommended only for patients older than 50 years with a significantly increased risk profile [4]. Standardized tools such as the Framingham Risk Score and the AGLA Guidelines should be used for risk stratification.
P2Y12 inhibitors
Clopidogrel: Clopidogrel (300-600 mg loading dose and 75 mg/d maintenance dose) requires oxidation by the hepatic cytochrome P450 system for conversion to the active metabolite. In this process, about 85% of the prodrug is hydrolyzed by esterases into an inactive form. The active metabolite selectively and irreversibly inactivates the P2Y12 receptor, preventing ADP-mediated platelet activation. While dual antiaggregatory therapy with aspirin and clopidogrel reduces the rate of ischemic events after PCI [10–11], up to 10% of patients on this combination therapy experience ischemic recurrence within the first year, with a rate of stent thrombosis of up to 2% [12]. This is attributed to the partly large interindividual variability of platelet reactivity, due to variable bioavailability. Pharmacogenetically, this is largely due to “loss of function” variants on the CYP 2C19 and especially C19*2 allele. Patients with this mutation exhibit lower levels of the active metabolite and correspondingly reduced platelet aggregation inhibition. While rapid genetic tests for this mutation are now more widely available, the EMA and FDA advise against their unselective use. Genetic testing and platelet function analysis should be reserved for patients with status post stent thrombosis on treatment, high risk of bleeding, and stents in critical vessels (eg, left main stem).
Interactions with PPIs, particularly omeprazole and esomeprazole, have been widely described. While pharmacodynamic interactions are considered relatively certain, clinical relevance could not be confirmed with certainty. In general, PPIs should not be prescribed automatically even in patients on dual antiaggregation, but should be given to high-risk groups with status post GI bleeding, concomitant anticoagulation, on steroid or NSAID therapy, older age (>65 years) or with H. pylori infection. In case of a necessary PPI therapy, pharmacodynamic studies argue for the use of newer substances (e.g. pantoprazole).
Prasugrel: Prasugrel (60 mg loading dose, 10 mg/d maintenance dose) is an irreversible inhibitor of the P2Y12 receptor with a faster onset of action and more potent effect than clopidogrel. In the TRITON-TIMI 38 trial, approximately 10 000 patients with ACS (STEMI or NSTEMI) and planned PCI were treated with either clopidogrel or prasugrel, started during or after the intervention [13]. After 15 months, recurrent cardiovascular events were significantly reduced in the prasugrel group (from 11.2% to 9.3%; relative risk (RR) 0.82; p=0.002). In particular, the risk of myocardial infarction was significantly lower with prasugrel (relative risk reduction of 23.9%). However, this came at the price of a significantly increased rate of major and fatal bleeding (2.4% vs. 1.8%; HR 1.4; p=0.02). Given the significantly lower rate of stent thrombosis (1.13% vs. 2.35%; HR 0.48; p<0.0001), prasugrel should also be considered especially in patients with stent thrombosis on clopidogrel. In patients undergoing PCI for ACS (STEMI or NSTEMI), prasugrel is preferred to clopidogrel in the absence of appropriate contraindications. These include status post CVI or TIA, as there is an unfavorable benefit-risk profile for this group. Similarly, prescribing should tend to be discouraged in those over 75 years of age and patients with low body weights of <60 kg. If it is still deemed necessary, maintenance therapy should be given at reduced doses of 5 mg/d.
Ticagrelor: Ticagrelor is a reversible P2Y12 inhibitor that additionally inhibits ADP reuptake via ENT-1 (Equilibrative Nucleoside Transporter). Plasma half-life is 6-12 h. The loading dose is 180 mg, followed by a maintenance dose of 2× 90 mg/d. Like prasugrel, ticagrelor has an accelerated and more predictable onset of action compared with clopidogrel and more rapid regeneration of platelet function after discontinuation. Metabolites of the CYP3A4 system such as simvastatin are increased in plasma levels by ticagrelor, whereas CYP3A4 inhibitors (e.g., diltiazem) increase ticagrelor levels. In the PLATO trial, approximately 18 000 patients with ACS were treated with either clopidogrel or ticagrelor [14]. At 12 months, a significant reduction in the primary endpoint of myocardial infarction, vascular death, and stroke was seen with ticagrelor (9.8% vs. 11.7%; HR 0.84; p=0.001). The rate of non-vascular mortality was also significantly reduced in the ticagrelor group (4% vs. 5.1%; p=0.001). The frequency of non-CABG-associated major bleeding was found to be significantly higher with ticagrelor (4.5% vs. 3.8%; p=0.03). The overall rate of fatal hemorrhage did not differ significantly between groups, although the rate of fatal intracranial hemorrhage was significantly increased with ticagrelor (0.1% vs. 0.001%; p=0.02). Ticagrelor was more associated with common side effects such as dyspnea without morphologic correlate (13.8% vs. 7.8%), ventricular pauses, and asymptomatic hyperuricemia. Like prasugrel, ticargrelor is preferred to clopidogrel therapy after ACS (1B).
Cangrelor: Cangrelor is an ATP analogue administered intravenously that reversibly binds to the P2Y12 receptor with high affinity. The plasma half-life is very short at ten minutes. It exerts potent inhibition of ADP-dependent platelet activation and allows rapid recovery of TC function as early as one to two hours after stopping the infusion. Three large-scale trials compared cangrelor – started before PCI – with clopidogrel, administered either before/after PCI or according to local practice (CHAMPION-PCI; CHAMPION-PLATFORM; CHAMPION-PHOENIX). Here, a meta-analysis [15] demonstrated a 19% reduction in the relative risk of periprocedural mortality, myocardial infarction, target vessel revascularization, and stent thrombosis with cangrelor. The relative risk for stent thrombosis alone was shown to be reduced by as much as 39%. This again came at the price of a significantly increased bleeding rate (cangrelor 0.9% vs. clopidogrel 0.6%; OR 1.38; p=0.007). Cangrelor is approved as an adjunct to PCI in patients without pretreatment with P2Y12 inhibitors and without GP IIb/IIIa receptor blockade.
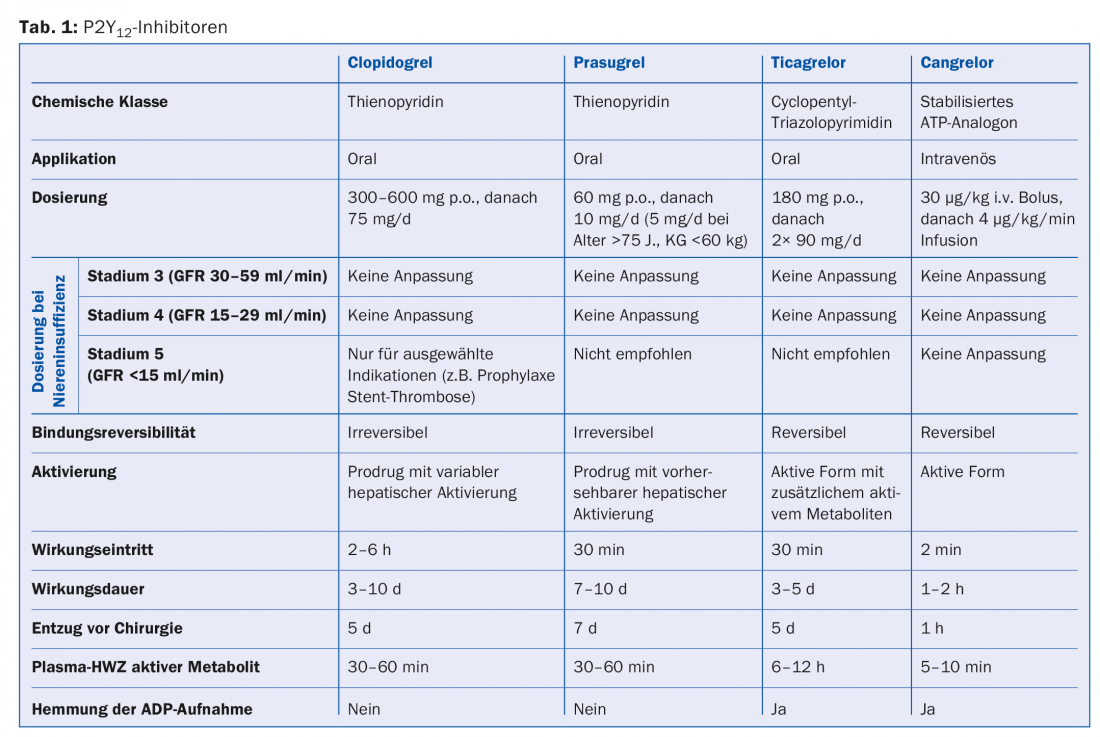
Table 1 provides an overview of P2Y12 inhibitors in use.
GP IIb/IIIa receptor inhibitors
Abciximab, eptifibatide, tirofiban: Intravenously applied GP IIb/IIIa inhibitors inhibit aggregation by blocking fibrinogen binding to activated GP IIb/IIIa receptors on adjacent platelets. A meta-analysis of six randomized clinical trials involving just over 29 000 patients showed a 9% reduction in the relative risk of death and nonfatal myocardial infarction with the addition of GP IIb/IIIa inhibitors to heparin therapy [16]. The positive effect was greatest in patients who underwent PCI. The risk of severe but not intracranial hemorrhage was found to be significantly increased. It should be noted here that most studies preceded the widespread use of P2Y12 inhibitors, and combinations with prasugrel and ticagrelor were never studied prospectively. Accordingly, GP IIb/IIIa inhibitors should be reserved for emergency situations only in combination with these drugs and also in patients on oral anticoagulation. In impaired renal function with a GFR <50 ml/min, eptifibatide requires dose adjustment, whereas this is only indicated for tirofiban from a GFR of <30 ml/min, and for abciximab the manufacturers recommend careful consideration of the benefit-risk ratio. For a GFR of 15-30 ml/min, only tirofiban is approved; below that, the use of all agents is discouraged.
PAR 1 receptor antagonist
Vorapaxar: Vorapaxar is an orally active selective inhibitor of platelet thrombin receptor PAR 1. In the TRACER trial, vorapaxar was compared with placebo in addition to standard therapy in nearly 13 000 NSTEMI patients. There was no reduction in the primary endpoint of cardiovascular death, MI, CVI, repeat ischemia, or repeat revascularization with vorapaxar (18.5% vs. 19.9%; HR 0.92; p=0.07). On the other hand, significantly more severe and intracranial hemorrhage occurred with vorapaxar (7.2% vs. 5.2%; HR 1.35; p<0.001). In the TRA 2P-TIMI 50 trial, vorapaxar was compared with placebo in addition to standard therapy in just over 24 000 patients with st n MI, st n CVI, or PAVK. Here, vorapaxar was associated with a moderate reduction in cardiovascular death, MI, and CVI over three years (9.3% vs. 10.5%; HR 0.87; p=0.001). This was again at the cost of significantly increased intracranial hemorrhage rates (1% vs. 0.5%; p<0.001). Accordingly, the drug is contraindicated in patients with cerebrovascular disease. In the United States, vorapaxar has gained approval for secondary prevention in MI, although the moderate benefit must be weighed carefully against the increased risk of bleeding.
Platelet aggregation inhibition after PCI – use according to clinical scenarios.
While platelet aggregation inhibition is standard therapy for atherosclerotic disease, the duration and intensity vary according to the urgency of the disease. Various scenarios are discussed below, and Table 2 provides an overview of these.
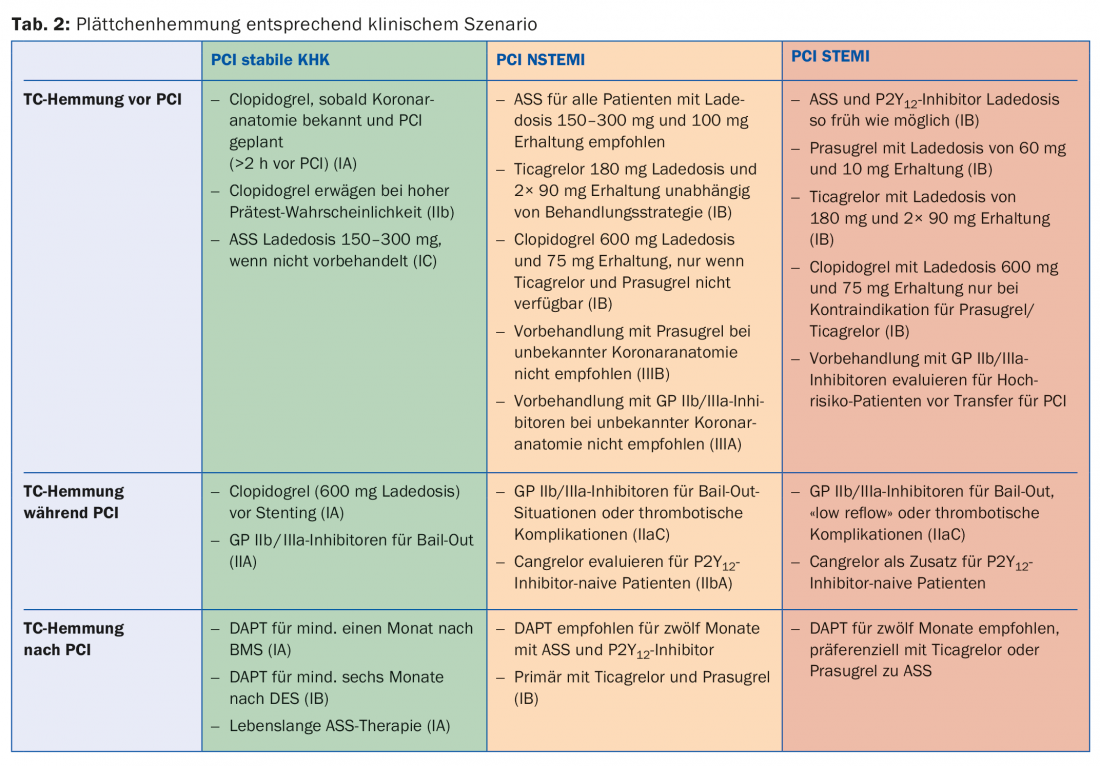
Stable coronary artery disease (SCAD): In general, it is recommended to use a radial approach and preferentially the latest generation of DES for PCI (1A). Dual antiplatelet therapy (DAPT) regularly consists of aspirin and clopidogrel in SCAD after PCI. All patients with SCAD or suspected SCAD should optimally be pretreated with aspirin 100 mg. If this is not the case, an oral loading dose of 150-300 mg must be applied before elective PCI. There is no evidence for pretreatment with clopidogrel before purely diagnostic PCI. However, if coronary anatomy is already known and revascularization is planned accordingly, a loading dose of clopidogrel 600 mg is recommended at least two hours before PCI. In patients not yet pretreated, a loading dose of 600 mg clopidogrel is recommended during PCI once the indication for stenting has been established. The use of GP IIb/IIIa antagonists under PCI should be reserved for emergency situations (eg, impending coronary thrombosis) in SCAD. After PCI, DAPT with clopidogrel 75 mg/d and aspirin 100 mg/d is recommended for 1 month after BMS implantation (1A) and for 6 months after DES implantation (1B). Thereafter, monotherapy usually with aspirin should optimally be continued for life (1A). It has been shown that the circumstances of interruption are important for the development of coronary complications after suspension of dual antiaggregation. In 50% of cases, abortion was prescribed by a physician and did not result in an increased risk of adverse events. In the 14% of cases where bleeding or malcompliance caused the interruption, the number of relevant cardiac complications increased substantially, although this effect wore off after 30 days [17]. Accordingly, good information and instruction of the patients is important.
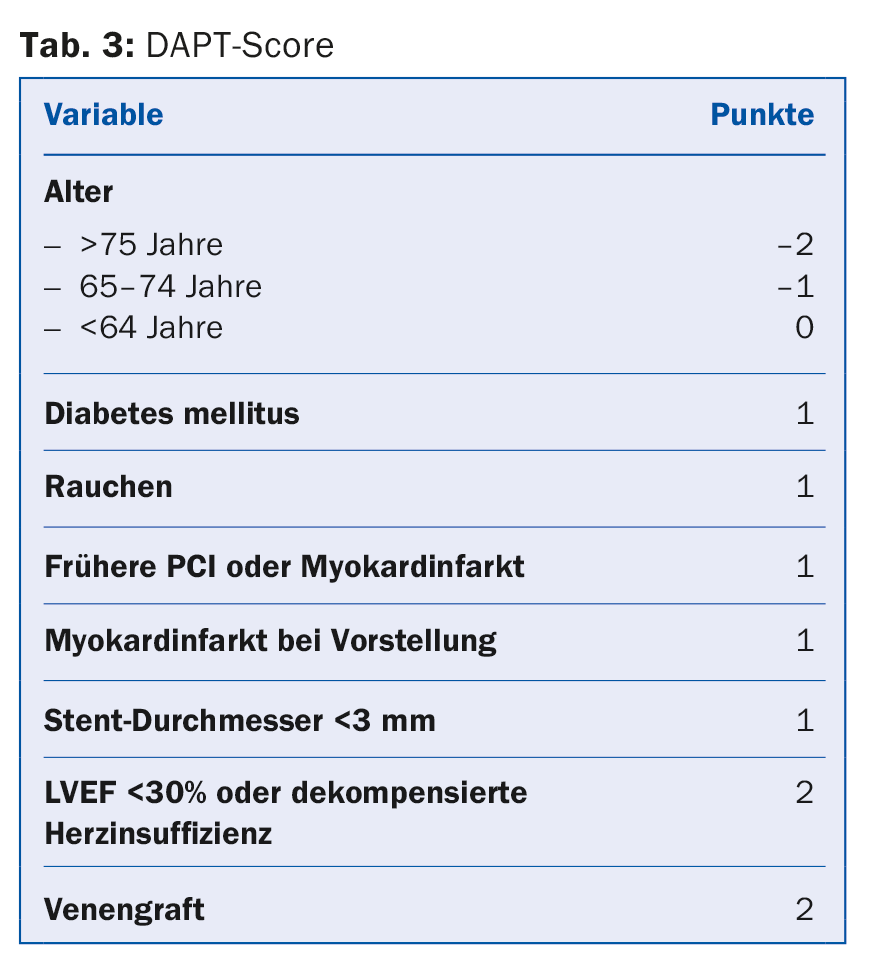
NSTEMI: DAPT after NSTEMI consists of aspirin and a P2Y12 inhibitor, preferentially ticagrelor or prasugrel (1B). Aspirin is recommended for all patients with a loading dose of 150-300 mg p.o. (or 80-150 mg i.v.) in aspirin-naive patients, followed by a long-term maintenance dose of 100 mg/d. A P2Y12 inhibitor is combined with aspirin for usually twelve months (1A). Ticagrelor with a loading dose of 180 mg followed by a maintenance dose of 2× 90 mg/d is recommended for all patients at moderate to high risk of ischemia, regardless of initial treatment strategy. This does not exclude patients who were pre-treated with clopidogrel, which must be suspended under ticagrelor. Prasugrel with a loading dose of 60 mg followed by a maintenance dose of 10 mg/d is recommended for patients with known coronary anatomy who are definitely undergoing PCI. Clopidogrel (loading dose 300-600 mg followed by 75 mg/d) should be used only in patients with contraindications to ticagrelor or prasugrel and on oral anticoagulation. GP IIb/IIIa inhibitors during PCI may be used in case of thrombotic complications or in so-called bail-out situations (“rescue operations,” e.g., large thrombus, slow or absent flow). Cangrelor may be considered as a complementary antiplatelet agent in patients without prior P2Y12 blockade and estimated to be at high risk. Although DAPT is generally recommended for 12 months, the duration can be shortened to three to six months or extended up to 30 months (IIA) in selected patients according to individual risk of bleeding and ischemia [4]. To facilitate decision making, the AHA recommends the use of the DAPT score in current guidelines (Tab. 3). This was designed in the DAPT study and validated in the PROTECT study. A score of -2 to 1 denotes patients whose bleeding risk is increased 2.5-fold compared with the protective anti-ischemic effect and who are accordingly more likely not to benefit from extended DAPT. In turn, patients with a score of >2 experience an eightfold benefit of avoided ischemia compared with bleeding risk and are more likely to benefit [18].
STEMI: Patients with STEMI undergoing primary PCI should receive DAPT with aspirin and a P2Y12 inhibitor in addition to parenteral anticoagulation as promptly as possible (IA). Aspirin with a loading dose of 300 mg p.o. is recommended for all patients regardless of possible pretreatment to ensure total blockade of TXA2 synthesis. Lifelong monotherapy with 100 mg/d is recommended in the absence of contraindications. A P2Y12 inhibitor should usually be combined with aspirin for 12 months after STEMI (IA). Prasugrel and ticagrelor (IB) are again generally preferred here, after a pooled analysis of just over 48 000 patients showed a survival advantage without significantly increased bleeding rates in STEMI patients under these agents [19]. It is important to remember that the newer and more potent P2Y12 inhibitors should be avoided in st. n. (hemorrhagic) stroke or moderate-to-severe hepatic impairment. Similarly, data on combination with (D)OAK are lacking and should be discouraged accordingly. Here, or even if the compounds are not available, clopidogrel at 600 mg loading and 75 mg/d maintenance dose continues to be used (IB). The use of GP IIb/IIIa inhibitors during PCI seems reasonable in the case of large thrombus, slow or absent flow, or thrombotic complications (IIC) and is commonly done internationally, although there are no large randomized trials on this. The so-called upstream application (i.e., already before PCI) of GP IIb/IIIa inhibitors is controversial, although it can be considered in high-risk patients who still need to be transferred initially (IIB). Cangrelor may be used as a bridge/supplement to PCI in patients without pretreatment with P2Y12 inhibitors and without additional GP IIb/IIIa inhibitors according to local practice.
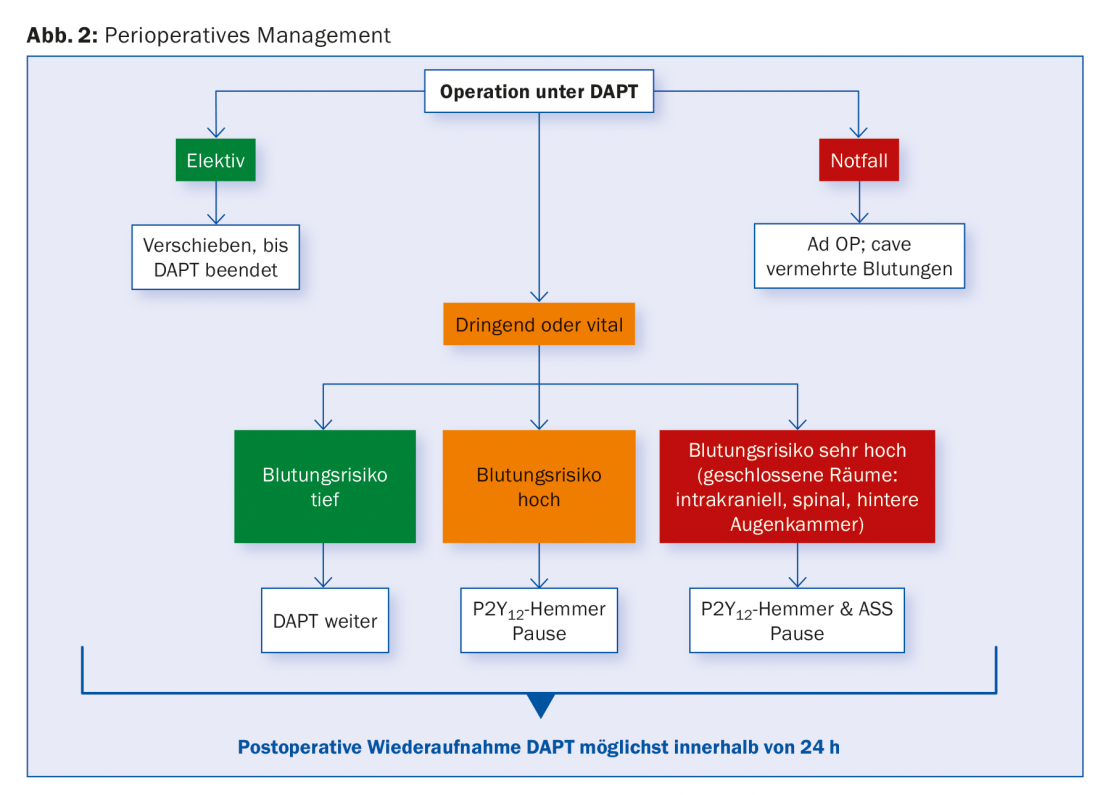
Surgery under DAPT
For patients with SCAD, DAPT is recommended for one to six months, depending on stent type, and for stent n. ACS, generally for 12 months.
It is not uncommon for a need for surgery to arise during this time period, either in terms of surgical revascularization (CABG) or non-cardiac in nature. Here, too, management depends on the individual risk of bleeding and ischemia and should be discussed on an interdisciplinary basis. For procedures with profound bleeding risk, continuation of DAPT should be sought. If this is not possible, at least aspirin should be left in place. Data from a large cohort study showed that the risk of relevant cardiac complications after surgical intervention was particularly increased in the first six months after PCI. Differences between stent types could not be found in this regard.
Elective surgical procedures should optimally be postponed until after completion of the recommended DAPT duration. For urgent procedures that require an interruption, it is recommended to pause clopidogrel and ticagrelor five days, prasugrel already seven days before, while continuing ASA. Data from CABG patients suggest that TC reactivity measurements may allow determination of a safe surgical time and shorten the pause by 50%. This would be a possible alternative to an arbitrary pause.
In high-risk patients, for example in the first days and weeks after stenting, bridging intravenous antiaggregation with GP IIb/IIIa inhibitors up to four hours before the procedure is sometimes suggested, although there are no prospective clinical data on this. Cangrelor was evaluated in the BRIDGE trial for bridging to CABG in patients pretreated with P2Y12 inhibitor. Cangrelor resulted in better preserved antiaggregation without increase in bleeding frequency [20]. None of the substances has yet received official approval in this area. Replacing a DAPT with LMWH or UFH is not appropriate. Postoperatively, resumption of all P2Y12 inhibitors as soon as possible is recommended. Figure 2 provides an overview.
Conclusion
Anticoagulation is a key principle in the treatment of coronary artery disease. Various platelet inhibitors and inhibitors of plasmatic coagulation are in daily clinical use. Application areas and combination options are expanding, such as the use of deep-dose rivaroxaban after ACS in addition to DAPT. The clinical success of individual preparations and their combinations is ultimately determined by whether the improved antithrombotic efficacy actually improves the prognosis without the possible benefit being offset by increased bleeding rates. Given the frequency of the disease and its major impact in leading cause of death statistics – and far from being limited to the Western world – it is important for the clinician to know the various agents with their indications, advantages and disadvantages in order to pursue the optimal strategy for an increasingly complex patient population.
Literature:
- Warren J: Remarks on angina pectoris. N Engl J Med Surg 1812; 1: 1-11.
- Stefanini G, et al: Drug-eluting coronary artery stents. N Engl J Med 2013; 368: 254-265.
- Roger VL, et al: Heart disease and stroke statistics – 2012 update: a report from the American Heart Association. Circulation 2012; 125(22): e1002.
- The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS): 2014 ESC/EACTS guidelines on myocardial revascularization. European Heart Journal 2014; 35: 2541-2619.
- Bonetti N, Beer JH: NOAK update – a small guide to a moving landscape. Practice Dispatch 2016; 12-19.
- The RISC Group: Risk of myocardial infarction and death during treatment with low dose aspirin and intravenous heparin in men with unstable coronary artery disease. The RISC group. Lancet 1990; 336: 827-830.
- Lewis HD Jr, et al: Protective effects of aspirin against acute myocardial infarction and death in men with unstable angina. Results of a Veterans Administration cooperative study. N Engl J Med 1983; 309: 396-403.
- Theroux P, et al: Aspirin, heparin, or both to treat acute unstable angina. N Engl J Med 1988; 319: 1105-1111.
- Cairns JA, et al: Aspirin, sulfinpyrazone, or both in unstable angina. Results of a Canadian multicenter trial. N Engl J Med 1985; 313: 1369-1375.
- Yusuf S, et al: Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med 2001; 345: 494-502.
- Mehta SR, et al: Effects of pretreatment with clopidogrel and aspirin followed by long-term therapy in patients undergoing percutaneous coronary intervention: the PCI-CURE study. Lancet 2001; 358: 527-533.
- Parodi G, et al: High residual platelet reactivity after clopi- dogrel loading and long-term cardiovascular events among patients with acute coronary syndromes undergoing PCI. JAMA 2011; 306: 1215-1223.
- Wiviott SD, et al: Prasugrel versus clopidogrel in patients with acute cor- onary syndromes. N Engl J Med 2007; 357: 2001-2015.
- Wallentin L, et al: Ticagrelor vs. clopidogrel in patients with acute coronary syndromes. N Engl J Med 2009; 361(11): 1045-1057.
- Steg PG, et al: Effect of cangrelor on periprocedural out- comes in percutaneous coronary interventions: a pooled analysis of patient-level data. Lancet 2013; 382: 1981-1992.
- Roffi M, et al: Platelet glycoprotein IIb/IIIa inhibition in acute coronary syndromes. Gradient of benefit related to the revascularization strategy. Eur Heart J 2002; 23: 1441-1448.
- Mehran R, et al: Cessation of dual antiplatelet treatment and cardiac events after percutaneous coronary intervention (PARIS): 2 year results from a prospective observational study. Lancet 2013; 382(9906): 1714-1722.
- Levine GN, et al: Focused Update Writing Group, 2016 ACC/AHA Guideline Focused Update on Duration of Dual Antiplatelet Therapy in Patients With Coronary Artery Disease. JACC 2016. doi:10.1016/j.jacc.2016.03.513 [Epub ahead of Print].
- Bellemain-Appaix A, et al: New P2Y12 inhibitors vs. clopidogrel in percutaneous coronary intervention: a meta-analysis. J Am Coll Cardiol 2010; 56(19): 1542-1551.
- Angiolillo DJ, et al: Bridging antiplatelet therapy with cangrelor in patients undergoing cardiac surgery: a randomized controlled trial. JAMA 2012; 307(3): 265-274.
HAUSARZT PRAXIS 2016; 11(6): 22-29