Infections in diabetic foot syndrome are common, cause high morbidity, and often require long hospitalizations with multiple surgical procedures, up to and including loss of a limb. The etiology is multifactorial, with peripheral vascular disease, neuropathy, and immunopathy playing the largest roles. Treatment needs a systematic and multidisciplinary approach. Classic clinical signs are often absent or appear only at advanced stages. Sampling from the wound and, if necessary, from the bone is necessary to start antimicrobial therapy. Surgical intervention is necessary for moderately to severely advanced wounds or systemic involvement. Then, debridement is the most important procedure and should involve all affected compartments. Amputation and/or wound closure should not be performed until the patient’s condition is stable and the treatment strategy is defined. Early diagnosis is very important to contain the extent of tissue damage. Postoperative care is essential to prevent recurrences and complications.
Infected foot ulcers in patients with diabetes mellitus are a major cause of morbidity, associated with reduced quality of life, need for specialized wound care, antimicrobial therapy, and frequent surgical interventions. Similarly, they are the most common cause of diabetes-associated hospitalizations and lower extremity loss. In a large, retrospective study of patients with diabetic foot ulcers, infection increased the risk of minor amputation by 50% compared with non-infected wounds. About 15% of patients with diabetes develop ulcerations of the feet during their lifetime and about two-thirds of these develop into complicated cases with osteomyelitis. Because of this, early diagnosis and an accurate treatment plan is paramount to avoid progression of the infection and amputation. In this short article, we will focus on the current treatment guidelines in diagnosis and treatment.
The three biggest risk factors are peripheral vascular disease, neuropathy, and immunopathy. More specifically, reduced or completely abolished sensitivity, combined with minor trauma, results in repetitive tissue damage. This leads to infection and poorer healing due to reduced blood flow and compromised immune system. The pathophysiology is simple: high blood glucose levels lead to metabolic changes in nerves (glycolization of proteins and decrease in axonal transport), reduce leukocyte phagocytosis, and therefore expose to infectious events. For this reason, a multidisciplinary approach is always necessary and involves a wide variety of disciplines: vascular surgery, angiology, orthopedics, plastic surgery, neurology, as well as nutritional counseling, physical therapy and, of course, internists and diabetologists.
Diagnosis
The diagnosis of soft tissue infections in such patients is not always easy, because the typical symptoms are often little pronounced or absent: The inflammatory response may be limited (ischemia and/or neuropathy) and often little or no pain is present (neuropathy). Systemic signs often occur late and in very severe cases when septic shock is already present or the condition is life-threatening. Local signs such as edema, hyperthermia, and pain (despite neuropathy) suggest infection. Microbiological sampling is then mandatory to confirm the diagnosis and establish therapy. Blood tests should always be performed (CRP, procalcitonin, leukocytes…). Highest sensitivity is often provided by uncontrolled hyperglycemia despite therapy.
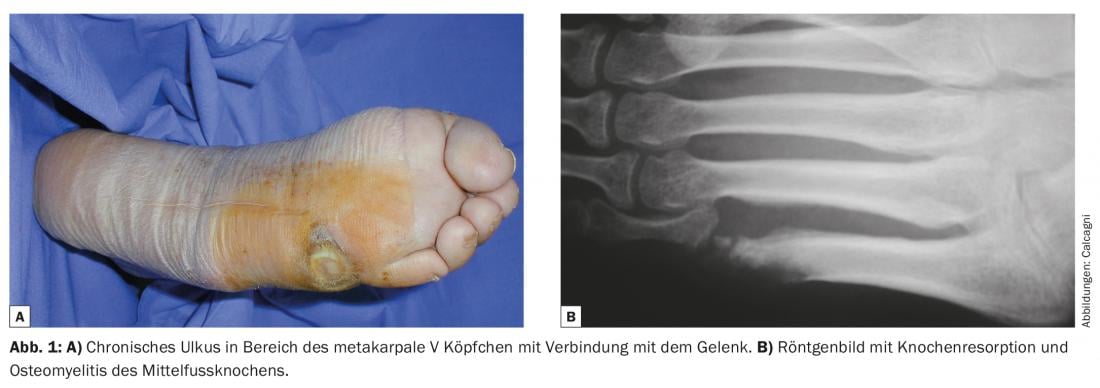
Additional diagnostics include a neurologic examination to look for concomitant neuropathy and/or peripheral nerve compression. As soon as bone involvement is suspected, an MRI examination should be performed; this should be done before starting antibiotic therapy, as a bone biopsy (for bacterial culture and histology) is necessary should signs of osteomyelitis be seen. Conventional radiographs are also necessary to rule out acute bone or joint problems (fractures, dislocations) or degenerative pathologies (such as Charcot arthropathy) (Fig. 1) . Finally, an angiological status should be obtained to assess arterial perfusion. For this purpose, noninvasive methods (Doppler) and, if peripheral revascularization is necessary, invasive diagnostics (CT or MRI angiography, direct angiography) are recommended.
Once the diagnosis has been made, classification into severity levels can help in planning the treatment strategy. Table 1 shows the Infectious Disease Society of America (IDSA) classification based on local clinical signs and the patient’s general condition.
Treatment
The treatment strategy depends on the severity of the infection. Table 2 shows the recommendations of the IDSA.
The patient should be stabilized regarding his diabetes, all other abnormal organ systems (heart, kidney…) need treatment as well. Antibiotic therapy should be adjusted to the antibiogram of the germs detected. There are a variety of recommendations in the literature on the duration and form of antibiotic therapy, but there is no clear evidence and therefore therapy should be based on clinical signs.

Should surgical debridement be indicated (Fig. 2), to achieve the best result, the surgeon must above all prevent the spread of infection and any progression of necrosis by incising all involved compartments, debridement of the necrotic areas, collection of bone samples for microbiology and histology, meticulous hemostasis, and intensive postoperative follow-up. The surgeon should be aware of the structures of the foot and lower leg compartments (deep and superficial) and the typical routes of spread of infection. This usually occurs along the tendons, which have the least tissue resistance. Infected joints and bones should be extensively debrided or amputated, depending on the local vascular situation.

The timing of surgery depends on the patient’s general condition. If septic shock is imminent, emergency incision and debridement must be performed. Definitive amputations can usually be planned secondarily. In most cases, repeated debridement is necessary to create clean and vital wounds. Negative pressure wound therapy (NPWT) is often very helpful in protecting wounds from drying and necrosis, but it cannot actually cure infection. Only accurate debridement, as often as necessary, can stabilize the situation and, together with an improvement in the accompanying circumstances, eventually lead to complete wound healing. However, negative pressure therapy is extremely good for reducing the size of wounds and stimulating granulation tissue. It is often the only way to achieve definitive wound closure in patients with a very poor vascular situation, although it may take months.
The last step is the definitive wound closure, which should comply with the rules of reconstructive plastic surgery. Superficial, small wounds usually heal secondarily; larger and deeper wounds may require split-skin or even complex flap dressings. Again, these decisions should be made in light of all factors (wound, age, vascular status, comorbidities).
Postoperative treatment
Treatment of diabetic foot ulcers is often long and difficult, so consistent treatment should continue after discharge from the hospital or an outpatient facility. Skin care, compression bandaging (if necessary), foot care and shoe adjustments are important to maintain the results achieved and to prevent relapse.
Literature:
- Ahmad J: The diabetic foot. Diabetes Metab Syndr. 2016; 10(1): 48-60. doi:10.1016/j.dsx.2015.04.002.
- van Baal JG: Surgical treatment of the infected diabetic foot. Clin Infect Dis. 2004; 39 Suppl 2(Suppl 2): S123-S128. doi:10.1086/383273.
- La Fontaine J, et al: Current concepts in the surgical management of acute diabetic foot infections. Foot (Edinb). 2014; 24(3): 123-127. doi:10.1016/j.foot.2014.05.003.
- Noor S, et al: Understanding Diabetic Foot Infection and its Management. Diabetes Metab Syndr. June 2016. doi:10.1016/j.dsx.2016.06.023.
- Wallace GF: Debridement of invasive diabetic foot infections. Clin Plast Surg. 2007; 34(4): 731-734. doi:10.1016/j.cps.2007.07.009.
- IDSA guidelines: Lipsky et.al: Diagnosis and treatment of diabetic foot infections, CID 2004: 39.
HAUSARZT PRAXIS 2016; 11(10): 27-30












