In 2018, approximately 10 million people contracted tuberculosis and 1.45 million died. An increasingly major challenge is the care of patients with multidrug-resistant tuberculosis (MDR-TB), for whom the two main drugs used in the standing therapy of tuberculosis – isoniazid and rifampicin – have lost their efficacy.
In 2018, approximately 10 million people contracted tuberculosis and 1.45 million died. An increasingly major challenge is the care of patients with multidrug-resistant tuberculosis (MDR-TB), for whom the two main drugs used in the standing therapy of tuberculosis – isoniazid and rifampicin – have lost their efficacy. Drug resistance is probably not infrequently due to differences in the pharmacokinetics of TB drugs [1]. However, in regions with a high proportion of MDR-TB in new diagnoses, direct transmission of the MDR pathogens is increasingly observed [2]. Therapy is lengthy and can be fraught with side effects, so an interdisciplinary and experienced team is needed to manage treatment. Treatment success for the multidrug-resistant form of tuberculosis was currently only 55-56% worldwide, according to the annual report of the World Health Organization (WHO) [2,3]. The low case-finding rate of MDR-TB worldwide remains problematic. Before a meaningful therapeutic decision can be made, diagnostics must be available that can be used to identify all relevant drug resistances, if possible. For example, of the 500,000 new MDR-TB cases estimated for 2018, only 187,000 were diagnosed and reported. At 156,000, the number of cases that could be assigned to treatment was correspondingly low. Since only about half successfully complete treatment according to WHO statistics, more than 80% of the estimated MDR cases remain mathematically uncontrolled and contribute to further spread [2].
In Germany, 118 cases with MDR-TB were reported in 2018, representing a stable share of 3.1% of new diagnoses over the past years. In the 2019 Robert Koch Institute (RKI) report, treatment results of MDR tuberculosis cases from 2017 are available and successful treatment was documented in only 39% of patients [4]. The cut-off date for the evaluation of these treatment results submitted to the RKI was March 1, 2019. However, in almost 45% of patients, therapy was not completed at the time of evaluation and the outcome of therapy was therefore unknown. In Switzerland, seven cases were diagnosed with MDR-TB in 2018 and 10 cases in 2019, according to the Federal Office of Public Health (Federal Office of Public Health, personal communication).
The following provides more detail on the 2019 and 2020 WHO recommendations for the diagnosis and treatment of drug-resistant TB, which have changed significantly.
Diagnostics
In patients in whom pulmonary tuberculosis is suspected on the basis of typical symptoms or on the basis of an abnormal chest radiograph, at least 2 good-quality sputum samples should be examined for mycobacteria, according to WHO recommendations [5,6]. In recent years, nucleic acid amplification tests (NAATs) for pulmonary and also extrapulmonary forms of TB have gained the highest importance in initial diagnostics. NAATs make it possible to distinguish M. tuberculosis complex from non-tuberculous mycobacteria (NTMs). With automated methods such as the Xpert® MTB/RIF, a test result for pathogen identification and rifampicin resistance can be expected after only a few hours. The new version of the Xpert® MTB/RIF Ultra showed improved overall sensitivity compared to the previous version (90% versus 85% [6]), especially also for paucibacillary pulmonary tuberculosis without microscopic detection of mycobacteria (sensitivity 77% versus 67% [7]). However, this improvement was accompanied by a deterioration in specificity (96% versus 99% [6]). Increased false positive results are possible, especially in patients with a history of TB in the past 5 years [7].
Microscopy of sputum to detect acid-fast rods provides an indication of the bacterial load of the sputum and thus the risk of infection. However, transmission is possible even with negative microscopy and positive NAAT [8]. Whenever tuberculosis is suspected, cultural culturing of the pathogen should be sought. Culture is currently still the standard for TB diagnostics. Compared to solid culture media, the liquid culture has a somewhat higher sensitivity, and the growth of mycobacteria can be detected about two weeks earlier due to the method.
Diagnosis of drug resistance
Despite methodological challenges, cultural or phenotypic resistance testing is currently still highly valued for clinical therapy decisions in Germany and Switzerland [9]. Phenotypic resistance testing of isoniazid, rifampicin, fluoroquinolones, aminoglycosides, and polypeptides appears to be more reliable here than for other antituberculosis drugs [10]. The reasons for this are on the one hand technical, such as the substance instability of the carbapenems. In some cases, threshold values for the categorization “sensitive”/”resistant” are missing or are not sufficiently substantiated scientifically. This applies in particular to the important substances cycloserine/terizidone and para-aminosalicylic acid (PAS) [11].
Pathogen cultivation and phenotypic resistance testing can take 2-10 weeks. During this time, calculated therapy would need to be implemented that is aligned with local resistance patterns [12,13]. The results of a molecular biological or genotypic resistance test are available much faster. In many cases, this can already be performed from direct material (e.g. sputum containing pathogens). The results should be available after a few hours to days and allow targeted therapy initiation. However, with molecular biology techniques, it is not currently possible to detect resistance to all available drugs. In addition, resistance not associated with gene mutations detected in rapid tests may be overlooked. Therefore, results of rapid molecular tests should usually be confirmed by phenotypic resistance testing. Increasingly, whole genome sequencing is providing additional results and is already replacing phenotypic resistance testing for first-line drugs in some countries [14,15].
New WHO diagnostic recommendations
Results of molecular biology testing show good agreement with phenotypic results for some drugs [15]. Therefore, since 2020, the WHO recommends molecular biological rapid tests in initial pathogen diagnostics as well as in resistance diagnostics in preference to microscopic and cultural methods. This applies in particular to the NAAT Xpert® MTB/RIF and the Xpert® MTB/RIF Ultra from Cepheid in pulmonary TB. Both tests indicate rifampicin resistance in addition to pathogen diagnosis. Alternatively, the Truenat® MTB, MTB Plus and MTB-RIF Dx tests from Molbio can be used, according to the WHO. For CSF diagnostics in suspected TB meningitis, a strong recommendation is made for the use of the Xpert® MTB/RIF Ultra as the initial test. For other specimen materials in cases of suspected extrapulmonary tuberculosis, the analyzed data justify only a weak recommendation primarily for the Xpert MTB/RIF [7].
So-called line probe assays (LPAs) such as those marketed by Hain Lifescience, for example, can be used to diagnose rifampicin and isoniazid resistance (GenoType® MTBDRplus) in microscopically or culturally positive sputum samples. LPAs for second-line drugs (GenoType® MTBDRsl) can be used in place of phenotypic testing to determine resistance to fluoroquinolones (FQs) and amikacin when rifampicin resistance has previously been identified. The same recommendation applies in cases of isoniazid monoresistance before initiation of therapy with fluoroquinolones. Phenotypic resistance testing continues to be recommended by WHO when testing with LPAs does not reveal resistance but the likelihood of additional resistance beyond MDR-TB appears high [6,7].
In the current year, the new NAAT rapid test Xpert® MTB/XDR for the simultaneous determination of resistance to isoniazid, fluoroquinolones, amikacin, kanamycin, capreomycin and ethionamide is to be evaluated by the WHO.
Sequencing
Whole genome sequencing (WGS) is a technique that can detect rare mutations associated with drug resistance. In addition, sequencing can be applied to analyze epidemiological data and trace the origin of tuberculosis disease outbreaks [16–18]. Comprehensive phenotypic and genotypic resistance testing including sequencing is offered by reference laboratories in Germany and should be performed at least for each MDR-TB strain. In this context, interpretation of gene mutations in terms of their clinical relevance is challenging in many cases and is an essential part of current research efforts to bring this technology into widespread use [19]. The reference laboratories in Germany and Switzerland now offer WGS.
Treatment of tuberculosis with isoniazid monoresistance.
Approximately 8% of TB worldwide are thought to show INH resistance without RMP resistance. Therefore, in 2018, WHO published a separate recommendation on therapy for INH monoresistance, recommending 4 -times therapy with levofloxacin together with or instead of INH for the entire therapy duration of 6 months [20,21]. This recommendation was adopted in 2020 [22,23].
In Germany, the proportion of INH-resistant strains was 9.4% in 2018 [4]; in Switzerland, the proportion was 6.2% of tested TB strains, according to the Federal Office of Public Health (Federal Office of Public Health, personal communication). The recommendation for the therapy of INH monoresistance (Tab. 1) will be re-evaluated in the already notified update of the German guideline. For the time being, however, the 2017 recommendations remain valid for treatment in Germany. An important prerequisite for treatment is the rapid and reliable testing of resistance to the fluoroquinolones often used in this situation.
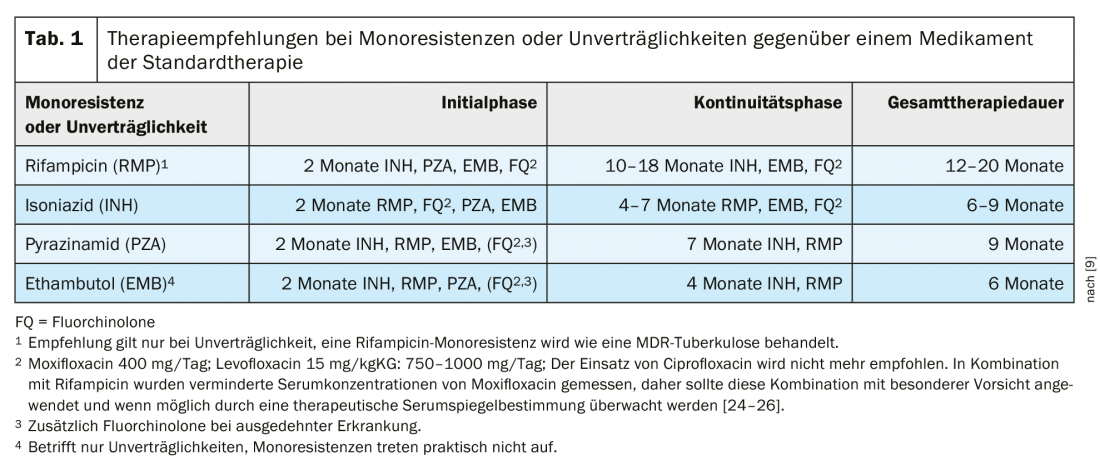
Treatment of tuberculosis with rifampicin monoresistance or intolerance.
WHO recommendations equate rifampicin monoresistance with MDR tuberculosis. Rifampicin resistance (RR) rarely occurs without isoniazid resistance. Because the absence of rifampicin has serious implications for treatment success, MDR and RR-TB are often combined in WHO documents. The new WHO recommendations comment that high-dose isoniazid (10 -15 mg/kgKG) may be used in MDR/RR as part of MDR therapy in the case of proven susceptibility or low-level resistance to isoniazid [21]. The current German therapy guideline recommends a different therapy for patients with rifampicin intolerance than for rifampicin resistance (Table 1).
Treatment of multidrug-resistant tuberculosis
WHO recommendations were initially updated in 2019 based on existing evidence [21]. In 2020, a full update of the recommendations followed with the associated Companion Handbook, which is intended to add practical aspects to the recommendations [22,23].
The drugs available for the treatment of drug-resistant TB have been classified into new groups by WHO since 2018 (Table 2). This classification remains valid with the new 2020 recommendations. In particular, the new drug bedaquiline, but also linezolid, are given a significantly higher priority with the new recommendations. In this context, the recommendation of completely oral therapy represents a real paradigm shift for most patients.
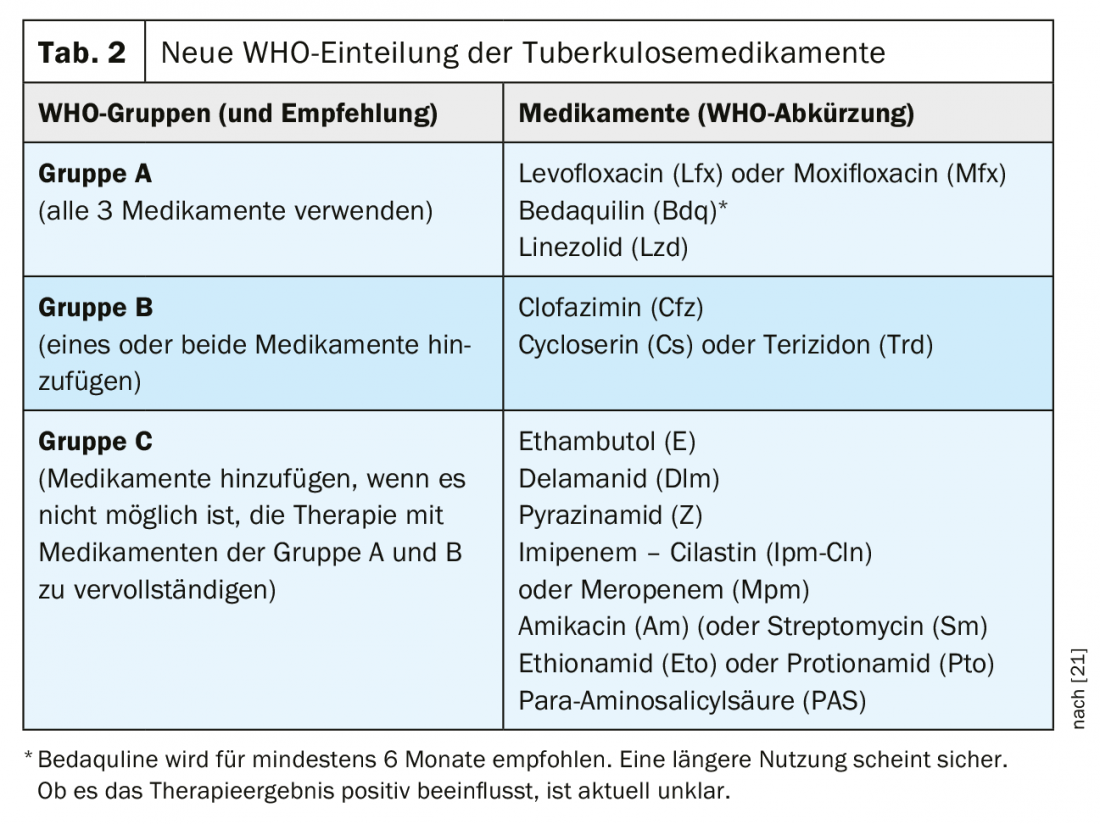
WHO recommends that a combination of at least four drugs with initial high probability of efficacy be assembled for MDR tuberculosis therapy [27].
- All 3 drugs of group A should be used if possible and supplemented by 1 drug of group B.
- If only 1 or 2 group A drugs can be used, then both group B drugs are supplemented.
- If it is not possible to compile a complete therapy from groups A and B, it should be supplemented by group C drugs.
The European Respiratory Society (ERS), American Thoracic Society (ATS), and Infectious Disease Society of America (IDSA) recommend at least five effective medications in the intensive phase, and four medications following [28].
Unfortunately, with the exception of fluoroquinolones, resistance testing for group A and B drugs is only established in reference laboratories. The new WHO recommendations continue to suggest a duration of 18-20 months for this longer therapy. However, it is commented that the duration of therapy can be adjusted according to the individual response to MDR-TB therapy. For most MDR-TB patients, a therapy duration of 15-17 months after culture conversion should be sufficient. If amikacin or streptomycin is used, the intensive phase with aminoglycosides should last 6-7 months in most cases [21].
A significant innovation is the downgrading of aminoglycosides in MDR-TB therapy. WHO recommends removing them from therapy if possible. Only amikacin (alternatively streptomycin) should still be used if there is no other way to complete MDR-TB therapy. Capreomycin and kanamycin are generally no longer recommended.
With the new WHO recommendations of 2020, the use of bedaquiline will now be
- beyond 6 months,
- simultaneously with the new drug delamanid
- or during pregnancy
considered to be sufficiently safe. The data base for these recommendations was limited, so it must be possible to monitor therapy well, especially in such situations [22,23].
Statement on the WHO recommendations from a German perspective
Following the update of the WHO recommendations in 2019, a joint statement was published by the Research Center Borstel (FZB) and the DZK supporting the new WHO recommendations.
For MDR treatment in Germany, initiation of MDR therapy consisting of five drugs with bedaquiline, linezolid, levo- or moxifloxacin, clofazimine, and terizidone is recommended after molecular biological evidence of rifampicin resistance and exclusion of fluoroquinolone resistance (gyrA gene position 90, 91, and 94 wild type). In the case of molecular evidence of fluoroquinolone resistance, an intravenous port system should be implanted and therapy with bedaquiline, linezolid, clofazimine, terizidone, amikacin, or meropenem/amoxicillin-clavulanic acid initiated. The goal remains to initially administer at least five effective substances. The choice of additional group C drugs should be made depending on the results of resistance testing [29].
Amikacin is thereby recommended for the treatment of adult patients with MDR/RR-TB only:
- when a therapeutic regimen cannot be composed of drugs from groups A and B,
- the results of resistance testing suggest efficacy of the drug and that close monitoring can be ensured to detect the development of side effects at an early stage [29].
Such monitoring is feasible in most specialized centers in Germany and Switzerland, so that amikacin remains a therapeutic option here. The new WHO recommendations of 2020 support this position under the conditions mentioned [22].
In Switzerland, there are no specific recommendations for the therapy of MDR-TB. Currently, the Federal Office of Public Health and the Lung League recommend consulting experts in case of rifampicin resistance. In this context, experts can be reached on weekdays from 8-12 and 14-17 via the toll-free telephone number of the Swiss Lung League 0800 388 388 . Led by them, there is an expert group of clinicians, microbiologists and public health specialists who discuss complex cases and answer questions from practitioners on an online platform.
Short-term therapy of multidrug-resistant tuberculosis.
Since 2016, WHO has recommended short-term therapy over 9-12 months with a fixed drug combination for selected MDR-TB patients, and with the new recommendations, favors this over longer individualized therapy [22,23,30]. The prerequisite for such short-term treatment is the proven sensitivity of the pathogen to fluoroquinolones and ideally to all other substances used. In addition, the patient must not have been previously treated with any of the drugs used for more than 1 month. In Europe, this is probably true for only a few patients, as a high proportion of MDR-TB cases have additional resistance [12,31]. Therefore, a preferential use of short-term therapy cannot be recommended in Germany at present [9,29].
The drug combination recommended by WHO in 2016 contained aminoglycosides, which should no longer be used. Now, recent data from South Africa showed that fully oral short-term therapy using bedaquiline improves treatment outcomes and leads to fewer treatment discontinuations [32]. Therefore, for patients with MDR-TB strains in whom fluoroquinolone susceptibility has been demonstrated and short-term therapy is indicated, WHO has recommended against aminoglycosides in favor of bedaquiline since 2020. Completely oral short-term therapy for 9 -12 months should include bedaquiline, levo- or moxifloxacin, ethionamide, ethambutol, pyrazinamide, high-dose isoniazid, and clofazimine [22,23].
New combination therapy for MDR-TB with fluoroquinolone resistance.
Bedaquiline, pretomanide, and linezolid (BPaL) combination therapy for 6-9 months has been approved in the U.S. since Aug. 14, 2019, for the treatment of extensively resistant tuberculosis (XDR-TB) and for MDR-TB treatment failure. The European Medicines Agency approved pretonamide in July 2020 for the treatment of XDR- TB or MDR- TB in the absence of other treatment options. The new nitroimidazole pretomanide was developed by the nonprofit organization TB Alliance, and the combination therapy was tested in 109 patients in South Africa. An open-label study without a comparator arm (Nix-TB) led to approval, demonstrating good efficacy in complicated resistance with a treatment success rate of 90% [33].
WHO compared data from the Nix TB trial with data from patients who received bedaquiline and linezolid as part of combination therapy for a mean of 21-26 months. Both groups showed a very good treatment success of 97% (BPaL) and 92% (comparison group) [23]. Analysis of the Nix-TB trial data showed that serious adverse events with BPaL may occur in 25% of patients. Linezolid was used in the Nix-TB study at a dosage of 1200 mg daily. This dosage is approved for other bacterial infections with shorter duration of therapy. Dose-related adverse drug reactions such as bone marrow depression and peripheral neuropathy occurred with corresponding frequency in the Nix-TB study. Following the experience of the study, linezolid dosing can be reduced to 600 to 300 mg after 4 weeks of high-dose therapy if side effects are relevant. Interruption of therapy for up to 35 days also appears possible. The total duration of therapy with BPaL should be at least 6 months. If sputum culture conversion has not been achieved after 4 months, the duration of therapy should be extended to 9 months [23].
The use of BPaL is recommended by WHO only in MDR-TB patients with fluoroquinolone resistance for whom therapy cannot otherwise be assembled. In addition, use should be under study conditions and prior therapy with bedaquiline or linezolid for more than 2 weeks should not have occurred [23].
Further studies aimed at improving the tolerability of the drug combination are currently being conducted. For example, the ZeNix trial is testing different doses of linezolid.
Conclusion and outlook
The global incidence of tuberculosis (TB) is currently falling at 2% per year. This decrease is far from sufficient to achieve the goals of the WHO EndTB strategy [2]. Drug resistance is a significant obstacle in this regard. The changes in WHO treatment recommendations for MDR-TB summarized here are the welcome result of increasing research in this area. In this context, the establishment of molecular diagnostics and the approval of three new drugs for the treatment of M/XDR-TB (bedaquiline, delamanid and pretomanid) represent major achievements. Yet, the number of MDR-TB cases estimated annually is not declining at a sufficient rate, and only 51% of TB cases confirmed globally were even tested for rifampicin resistance in 2018.
In Germany and Switzerland, MDR-TB case numbers are low and sufficient resources are available. Therapy is individualized after extensive resistance testing and can lead to improved therapeutic outcomes [34,35]. However, the greatest challenges to TB control in the 21st century primarily involve high-incidence countries with limited resources. In addition to innovative further developments in diagnostics and therapy, prevention and, in particular, the development of an effective vaccine will be of decisive importance in the fight against tuberculosis. There is an urgent need to improve global access to existing and new diagnostic and therapeutic options for everyone.
Take-Home Messages
- Tuberculosis is a rare disease in Switzerland, but 10 million new cases of tuberculosis are diagnosed each year worldwide.
- In multidrug-resistant tuberculosis, the two main drugs used in tuberculosis therapy, isoniazid and rifampicin, are no longer effective.
- Therapy in Germany and Switzerland is individualized after extensive resistance testing.
- Molecular biological methods enable rapid diagnostics for the initiation of targeted therapy. At present, the results should still be confirmed by phenotypic testing.
- The complex and lengthy therapy of MDR tuberculosis should be managed by experienced experts. Free counseling services are available from the Swiss Lung League (Tel.: 0800 388 388).
Literature:
- Prideaux B, et al: The association between sterilizing activity and drug distribution into tuberculosis lesions. Nat Med 2015; 21(10): 1223-1227.
- Global tuberculosis report 2019. Geneva: World Health Organization 2019. Licence: CC BY-NC-SA 3.0 IGO.
- Global tuberculosis report 2018, World Health Organization Geneva.
- Report on the epidemiology of tuberculosis in Germany for 2018. Robert Koch Institute 2019.
- Mase SR, et al: Yield of serial sputum specimen examinations in the diagnosis of pulmonary tuberculosis: a systematic review. Int J Tuberc Lung Dis 2007; 11(5): 485-495.
- WHO operational handbook on tuberculosis. Module 3: diagnosis – rapid diagnostics for tuberculosis detection. Geneva: World Health Organization 2020. Licence: CC BY-NC-SA 3.0 IGO.
- WHO consolidated guidelines on tuberculosis. Module 3: diagnosis – rapid diagnostics for tuberculosis detection. Geneva: World Health Organization 2020. Licence: CC BY-NC-SA 3.0 IGO.
- Tostmann A, et al: Tuberculosis transmission by patients with smear-negative pulmonary tuberculosis in a large cohort in the Netherlands. Clin Infect Dis 2008; 47(9): 1135-1142.
- Schaberg T, et al: Tuberculosis Guideline for Adults – Guideline for Diagnosis and Treatment of Tuberculosis including LTBI Testing and Treatment of the German Central Committee (DZK) and the German Respiratory Society (DGP). Pneumology 2017; 71(6): 325-397.
- Lange CI, Abukabar I, Alffenaar JW: Management of patients with multidrug-resistant/extensively drug-resistant tuberculosis in Europe: a TBNET consensus statement. 2014.
- WHO, Technical report on critical concentrations for TB drug susceptibility testing of medicines used in the treatment of drug-resistant TB. 2018.
- Otto-Knapp R, et al: Resistance to second-line drugs in migrants with multidrug-resistant tuberculosis in the Berlin region. Pneumology 2014; 68: 496-500.
- Lange C, et al: Management of patients with multidrug-resistant/extensively drug-resistant tuberculosis in Europe: a TBNET consensus statement. The European Respiratory Journal 2014; 44: 23-63.
- Public Health England National Infection Service. National Mycobacterium Reference Service-South (NMRS-South). User handbook 2019.
- Allix-Beguec C, et al: Prediction of Susceptibility to First-Line Tuberculosis Drugs by DNA Sequencing. N Engl J Med 2018; 379(15): 1403-1415.
- Galagan JE: Genomic insights into tuberculosis. Nat Rev Genet 2014; 15(5): 307-320.
- Nikolayevskyy V, et al: Whole genome sequencing of Mycobacterium tuberculosis for detection of recent transmission and tracing outbreaks: A systematic review. Tuberculosis (Edinb) 2016; 98: 77-85.
- Merker M, et al: The Evolution of Strain Typing in the Mycobacterium tuberculosis Complex. Adv Exp Med Biol 2017; 1019: 43-78.
- Meehan CJ, et al: Whole genome sequencing of Mycobacterium tuberculosis: current standards and open issues. Nat Rev Microbiol 2019; 17(9): 533-545.
- WHO, WHO treatment guidelines for isoniazid-resistant tuberculosis: Supplement to the WHO treatment guidelines for drug-resistant tuberculosis 2018; World Health Organization: Geneva.
- WHO, WHO consolidated guidelines on drug-resistant tuberculosis treatment 2019; World Health Organisation Geneva
- WHO consolidated guidelines on tuberculosis. Module 4: treatment – drug-resistant tuberculosis treatment. Geneva: World Health Organization 2020. Licence: CC BY-NC-SA 3.0 IGO.
- WHO operational handbook on tuberculosis. Module 4: treatment – drug-resistant tuberculosis treatment. Geneva: World Health Organization 2020. Licence: CC BY-NC-SA 3.0 IGO.
- Weiner M, et al: Effects of rifampin and multidrug resistance gene polymorphism on concentrations of moxifloxacin. Antimicrob Agents Chemother 2007; 51(8): 2861-2866.
- Nijland HM, et al: Rifampicin reduces plasma concentrations of moxifloxacin in patients with tuberculosis. Clin Infect Dis 2007; 45(8): 1001-1007.
- Alsultan A, Peloquin CA: Therapeutic drug monitoring in the treatment of tuberculosis: an update. Drugs 2014; 74(8): 839-854.
- Ahmad N, et al: Treatment correlates of successful outcomes in pulmonary multidrug-resistant tuberculosis: an individual patient data meta-analysis. Lancet 2018; 392(10150): 821-834.
- Nahid P, et al: Treatment of Drug-Resistant Tuberculosis. An Official ATS/CDC/ERS/IDSA Clinical Practice Guideline. Am J Respir Crit Care Med 2019; 200(10): e93-e142.
- Maurer B, et al: Joint statement on the new WHO recommendation on the treatment of multidrug-resistant and rifampicin-resistant tuberculosis. Pneumology 2019; 1-4 (in press).
- WHO Treatment guidelines for drug-resistant tuberculosis – Update 2016.
- Günther G, et al: Multidrug-resistant tuberculosis in Europe, 2010-2011. Emerging Infectious Diseases 2015; 21: 409-416.
- Rapid communication: key changes to treatment of drug-resistant tuberculosis. Geneva: World Health Organization 2019 (WHO/CDS/TB/2019.26). Licence: CC BY-NC-SA 3.0 IGO.
- Conradie F, et al: Treatment of Highly Drug-Resistant Pulmonary Tuberculosis. New England Journal of Medicine 2020; 382(10): 893-902.
- Olaru ID, et al: Bedaquiline-based treatment regimen for multidrug-resistant tuberculosis. Eur Respir J 2017; 49(5).
- Heyckendorf J, et al: Relapse-free cure from multidrug-resistant tuberculosis in Germany. Eur Respir J 2018; 51(2).
InFo PNEUMOLOGY & ALLERGOLOGY 2020: 2(3): 6-11.