Rhinosinusitis is divided into two groups: chronic with nasal polyps (CRSwNP) and those without (CRSsNP). It is a common disease, affecting about 11% of the population throughout Europe. Therapeutic options have been extremely poor until now, but this is likely to change with the approval of biologics.
Patients with CRSwNP are relevantly limited, which can be seen in studies of the quality of life of these sufferers, the extent of limitations is about on a par with heart failure, asthma or COPD. “We know that there are relevant influencing factors,” Prof. Dr. Martin Wagenmann, head of the focus on rhinology, allergology and endoscopic skull base surgery at the University Hospital Düsseldorf (D), explained by way of introduction. Among these comorbidities, he included asthma, sensitivity to ASA and nonsteroidal analgesics, colonization with Staph. aureus and allergic diseases.
The range of therapeutic options has been frighteningly small. Treatment with intranasal steroids represents the permanent and basic drug therapy. This is an effective form of therapy that can help the vast majority of these patients very well, but is still too rarely used in practice, as Prof. Wagenmann emphasized.
Antibiotics out of place in CRS
Nasal douches with saline solutions are also recommended, although the symptom relief that can be achieved with them is of course nowhere near as effective. If all this does not help, the only escalation so far has been systemic steroids. Antibiotics, on the other hand, are not suitable for the treatment of chronic rhinosinusitis, even though prescription figures and clinical reality still show otherwise, to the expert’s regret. However, he also referred to the necessary treatment of the concomitant diseases mentioned.
If drug therapy does not work at all, the option of surgical intervention remains; the gold standard here is endoscopic paranasal sinus (NNH) surgery. However, especially in the case of nasal polyps, this is associated with very high recurrence rates, particularly in those patients who have undergone surgery precisely for these polyps. In this regard, there is a meta-analysis of 45 studies involving 34220 patients with endoscopic NNH surgery for CRSwNP [1]. The average follow-up duration was approximately seven and a half years (89.6 months). One cannot assess from this meta-analysis how many patients actually experienced polyp recurrence. But it is possible to assess how many of the patients had to be re-operated for the same condition: this was the case in almost one in five (18.6%). The factors that most increased the risk of recurrence of surgery were:
- Fungal sinusitis (Allergic Fungal RhinoSinusitis, AFRS, 28.7%)
- Aspirin intolerance (Non-steroidal anti-inflammatory drug Exacerbated Respiratory Disease, NERD, 27.2%).
- Asthma (22.6%)
- prior polypectomy (26.0%)
- OP before 2008 (22.7%)
So there is obviously a clinical problem if surgical treatment produces so many treatment failures, Prof. Wagenmann summarized the figures.
Pathogenesis
Type 2 inflammation – i.e., increased production of cytokines IL-4, -5, and -13 in particular, activation of Th2 cells, of eosinophils, and also increased production of IgE – also appears to play a major role in chronic rhinosinusitis with nasal polyps. Studies suggest that, at least in Europe and North America, at least 80% of patients or their tissues have features of type 2 inflammation. These are precisely the cytokines for which it is now possible for the first time to take targeted therapeutic action (Tab. 1).
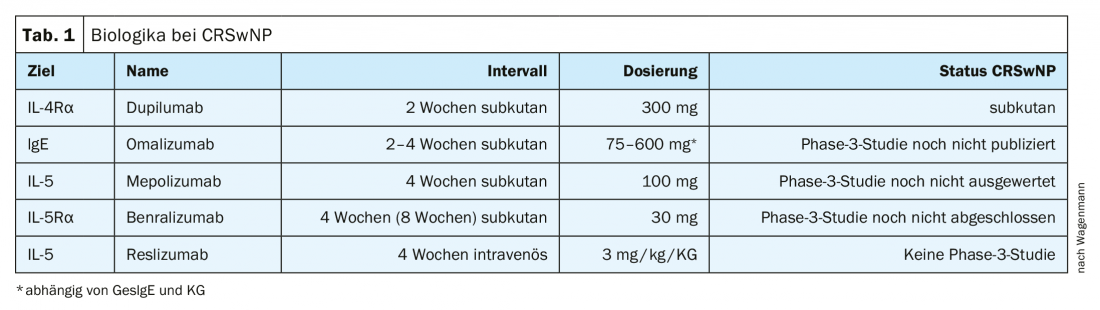
Until recently, there was very little or no information regarding biologics. Meanwhile, dupilumab, an antagonist against interleukin-4-Rα, has also been approved for treatment against CRSwNP since fall 2019. For omalizumab, a Phase 3 study has been completed but not yet fully published, a mepolizumab study has been completed and is currently being analyzed, and for benralizumab a Phase 3 study is nearing completion. Only for reslizumab is there currently no phase 3 study. “Finally, there is some movement here,” Prof. Wagenmann explained, backing up his optimism with promising study results.
The phase 3 study of dupilumab [2] actually consists of two multicenter randomized trials – SINUS-24 (n=276) for 24 weeks and SINUS-52 (n=448) for 1 year – in which dupilumab 300 mg or placebo was administered every 2 weeks (SINUS-52: every 4 weeks starting at week 25) in addition to continuous therapy with intranasal steroids (mostly mometasone). Only patients with severe chronic rhinosinusitis with nasal polyps who had already undergone unsuccessful surgery or one or more unsuccessful attempts at therapy with additional systemic steroids were included. Primary endpoints were polyp score, nasal obstruction, and CT score.
Significant improvements
The polyp score was determined by measuring the size of polyps relatively objectively by endoscopy of the nose. The objectivity of this parameter can also be seen in the placebo curves, which remained more or less constant over one year – i.e. there was no placebo effect on polyp size (Fig. 1) . In contrast, therapy with Dupilumab leads relatively quickly, clearly and highly significantly to a drastic reduction in polyp size. In the first study, in which the medication was stopped after 24 weeks, one can also read another important piece of information: if one stops the therapy, the polyps unfortunately also grow again.

If, on the other hand, the injection is continued (again divided into a 2- or 4-week injection period – ultimately 300 mg was allowed biweekly), then it can be seen that the polyp scores continue to decrease significantly, although not as rapidly as at the beginning. In addition, the symptoms also improved, most importantly for the patients, in addition to the reduction in smell, certainly the nasal obstruction – here, too, the improvement was just as clear and significant under dupilumab in both studies.
For Prof. Wagenmann, the most important finding was that more than 40% of patients in the placebo arm had to be treated with oral steroids or surgery during the course of the study, while the proportion of dupilumab patients who needed this was significantly lower at only 12.5% (Fig. 2). “That’s exactly what we want to avoid: these patients needing systemic steroid therapy or repeat surgery.”

Ultimately, all parameters measured in the dupilumab study were significantly improved. In the phase 3 study with omalizumab (whose study design is almost identical to that of the dupilumab study), very similar results are emerging, the only difference here: The time to first use of systemic steroids or surgery is not statistically significant.
Indication
Identifying the right patients for the use of biologics is definitely a challenge. Bilateral nasal polyps are a basic requirement, and at least three other criteria should be added, such as evidence of type 2 inflammation (the problem here is that “we don’t have good laboratory parameters that allow us to determine this,” says Prof. Wagenmann), the need for systemic steroids, restriction of quality of life, diagnosis of comorbid asthma, and – especially in asthmatics, the first sign to ask about – olfactory impairment.
In conclusion, the expert always advised close, interdisciplinary cooperation, because ENT physicians, for example, often do not recognize that patients with chronic rhinosinusitis with nasal polyps may have asthma that has not yet been diagnosed or adequately treated.
Source: Allergo Update, Berlin (D)
Literature:
- Loftus CA, et al: Int Forum Allergy Rhinol 2020; 10(2): 199-207.
- Bachert C, et al: Lancet 2019; 394: 1638-1650.
InFo PNEUMOLOGY & ALLERGOLOGY 2020; 2(2): 26-27 (published 6/17/20, ahead of print).