Status epilepticus is defined as an epileptic seizure with a duration of >5 min or a series of seizures between which the original neurological status is not regained. One of the most important options for the primary treatment of status epilepticus is the rapid and adequate administration of benzodiazepines, which are available in various dosage forms.
You can take the CME test in our learning platform after recommended review of the materials. Please click on the following button:
Status epilepticus is defined as an epileptic seizure lasting >5 min or a series of seizures between which the original neurological status is not regained. With an incidence of 10-40 per 100,000 person-years and a mortality rate of between 7 and 33%, status epilepticus (SE) is one of the most common neurological emergencies, but it is also an acutely life-threatening condition [1,2]. At 8.2%, mortality in patients without impaired consciousness is significantly lower than in patients with impaired consciousness (33%) [2]. Long-term consequences can include neurological, cognitive and behavioral disorders and a significant reduction in quality of life. In addition, status epilepticus can be associated with a significant deterioration in clinical outcome. Complications such as fractures, immobility, aspiration pneumonia acquired during the status, but also a restriction of everyday life skills as a result of a prolonged recovery process after congestion or loss of function due to a long stay in the intensive care unit play a role here. The outcome after status epilepticus is primarily determined by the etiology of the status epilepticus, the type or stage of the status epilepticus, its duration and also the age of the patient [3]. Predictors for recurrent SE were age <4 years, female gender, lack of drug responsiveness at 1st dose and symptomatic and progressive etiologies.
Guideline-based therapy of status epilepticus
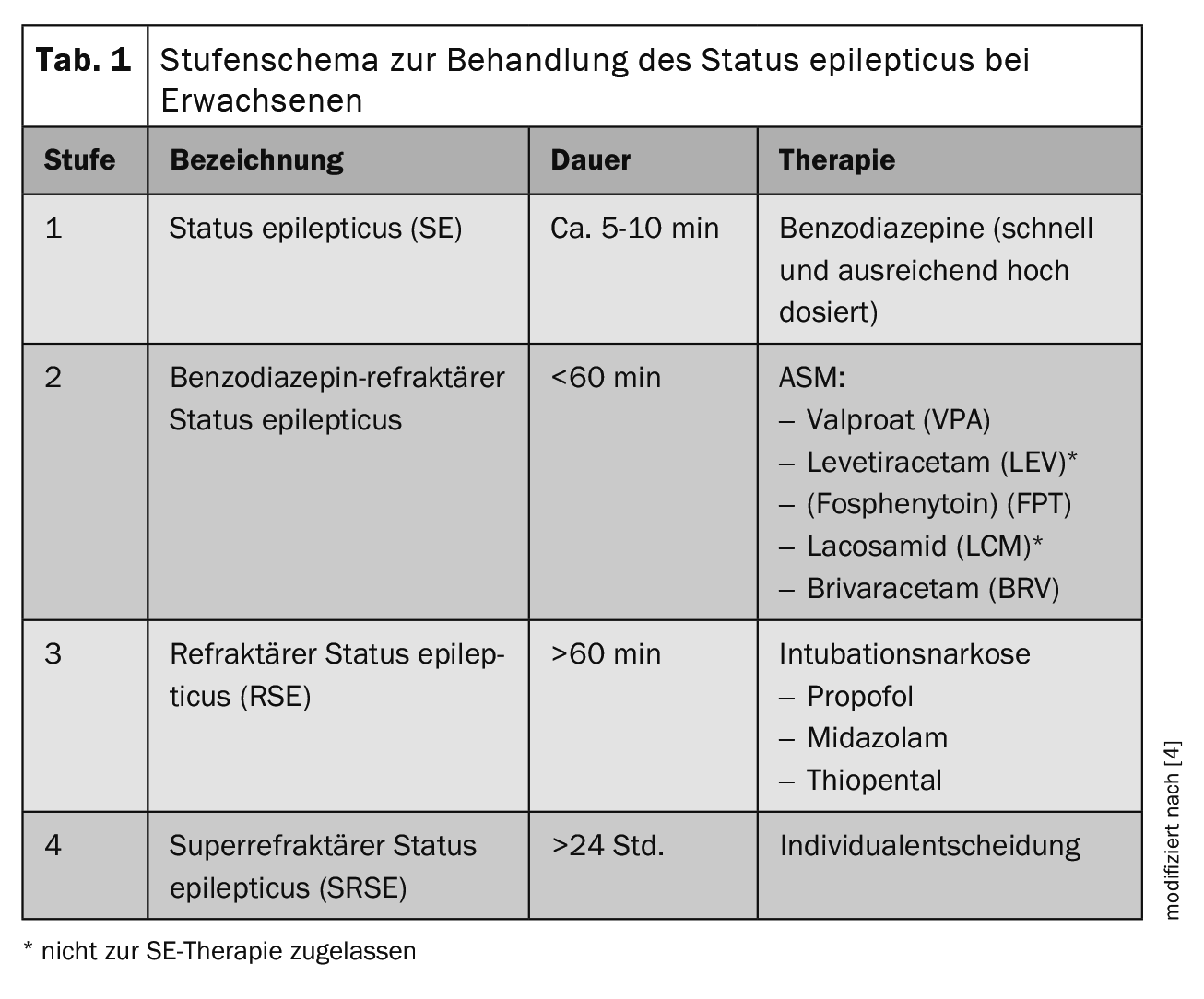
The success of treatment for SE is time-critical and depends on prompt neurological therapy and diagnostics. The preclinical phase is therefore of great importance in the provision of care. The treatment of SE in adults is divided into a step-by-step scheme in the current guideline (Table 1) [4]. After general measures such as ensuring the vital signs (ABCDE scheme), protecting the head from injury, administering O2 at an O2 saturation of <95% and a symptomatic reduction in temperature to >37.5°C, the initial therapy (stage 1) consists of administering benzodiazepines. The initial doses in adults >40 kg body weight (bw) are: Lorazepam 0.1 mg/kg bw (max. 4 mg/bolus administration, repeat 1× if necessary) or clonazepam 0.015 mg/kg bw (max. 1 mg/bolus administration, repeat 1× if necessary) or midazolam 0.2 mg/kg bw (max. 10 mg/bolus administration intramuscularly (i.m.), intravenously (i.v.) or intranasally (i.n.), repeat 1× if necessary) or diazepam 0.15-0.2 mg/kg bw (max. 10 mg/bolus administration, repeat 1× if necessary). For patients without IV access, midazolam should be administered intramuscularly by applicator or intranasally (10 mg for >40 kg, 5 mg for <40-13 kg bw) as a single dose). If the SE persists after the initial administration of a benzodiazepine, it should be checked whether the dose was adequate, as underdosing of the initial treatment is common due to fear of side effects inherent in the treatment with benzodiazepines and can result in reduced seizure control. If necessary, the benzodiazepine should be administered again in a sufficiently high dose as part of the initial therapy.
If the initial dose of benzodiazepine was sufficiently high, the dose should be increased within 30 minutes. in the 2nd level of therapy i.v. available seizure suppressants (ASM). As medicines of the 1st choice should be levetiracetam (LEV, 60 mg/kg bw, max. 4500 mg over >10 min i.v.) (not approved for SE therapy), valproate (VPA, 40 mg/kg bw, max. 3000 mg over >10 min i.v.) or fosphenytoin (FPHT, 20 mg/kg bw, max. 1500 mg over >10 min i.v.) [4]. Although fosphenytoin is approved in Germany and Austria, it is not marketed there and is not approved in Switzerland, so this is not relevant in the practical implementation of the therapy in German-speaking countries. Another possible alternative is the administration of lacosamide in a dose of 5 mg/kg i.v., which can be administered over 15 minutes [5]. However, it should be noted that a contraindication in the case of an AV block 2. or 3rd degree exists. Here, too, there is no approval for SE therapy. Initial case series and case reports describe the successful use of intravenously administered brivaracetam in refractory SE [6].
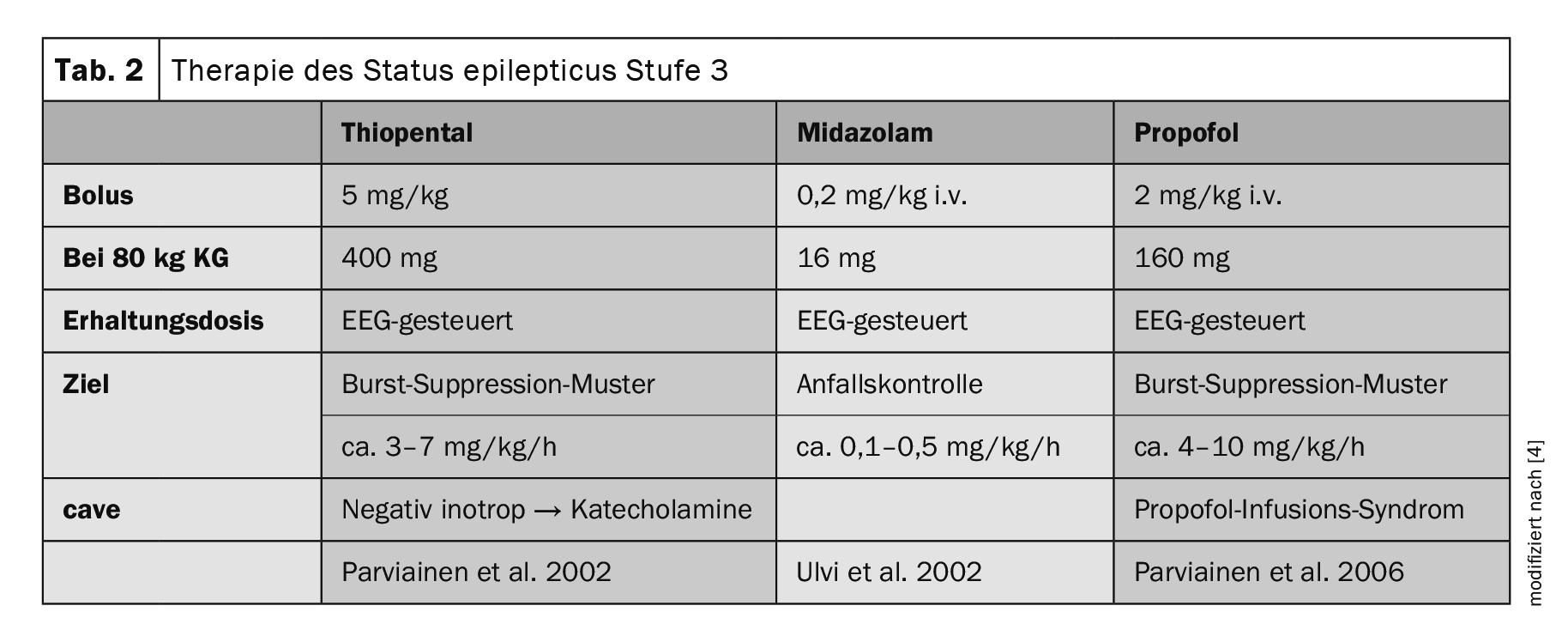
Following stage 2 treatment, intubation anesthesia with either thiopental, midazolam or propofol is performed if intravenous ASM fails (Table 2) [5]. Should this level of therapy also fail, the current guideline suggests further treatment options, although these are largely based on individual case reports. At this point, the SE is referred to as super refractory (SRSE). In addition to the administration of barbiturates, ketamine, NMDA antagonists, inhalation anesthetics such as isoflurane or desflurane, the enteral administration of other “classic”, only orally available ASMs or individual therapy trials with lidocaine, ketogenic diet and epilepsy surgery can be considered.
Current problems in the treatment of status epilepticus
Rapid and sufficiently high-dose treatment of SE is of great prognostic importance. The initial therapy in particular often deviates from the recommendations of the guideline. Guterman et al. 2021 were able to show that the treatment of preclinical SE rarely complied with the specified guidelines. Of the 9176 prehospital admissions for status epilepticus in 743 facilities, 7665 patients (83.6%) were treated with midazolam, 1264 (13.8%) with lorazepam and 245 (2.7%) with diazepam. There were 357 (3.9%; 95% CI: 3.5%-4.3%) cases in which the initial treatment was in line with expert recommendations in terms of recommended dose and type. The majority of patients therefore received lower doses of benzodiazepine than recommended [7].
In the SENSE study, a trinational cohort study, Kellinghaus et al. reported that in 15% of all cases no benzodiazepine was administered in the first stage of treatment. The data showed that guideline-compliant use of benzodiazepines was associated with treatment success and a significantly higher number of breakthrough SE [8].
In summary, all studies have shown that prehospital administration of benzodiazepines shortens the time to seizure control and reduces the length of hospital stay in patients with SE.
To optimize initial therapy, the simplified administration of benzodiazepines through the approval of injectors and nasal/buccal applications simplifies the initial administration by rescue workers and home caregivers, so that initial therapy can be further optimized in the future [9].
Gawedzki et al. were able to show in 2022 in a retrospective, monocentric observational study in the emergency department that the presence of an accompanying pharmacist in the status epilepticus team reduced the median time to administration of the 1. and 2nd ASM significantly reduced. In addition, the group of patients with a pharmacist present received a higher median dose of lorazepam equivalents (2.5 mg [IQR 2–4] vs. 2 mg [IQR 2–2]; p=0.04) and were more likely to receive a sufficiently high initial dose of at least 4 mg lorazepam equivalents (38% vs. 0%; p=0.11). However, there were no differences in hospital LOS or 30-day mortality [10]. It was concluded from this that the presence of a pharmacist or a therapy observer increases awareness of guideline-compliant therapy.
Another clinically relevant factor is the timely recognition of non-convulsive status epilepticus (NCSE), as delayed diagnosis and treatment of SE leads to increased mortality. Non-convulsive SE is one of the most frequently overlooked neurological emergencies, also because it is often associated with serious internal diseases that make diagnosis difficult [11]. The incidence study by Leitinger et al. showed that non-convulsive SE is associated with a high mortality rate (CFR 27.65%). With an incidence of 12.1/100,000, it is a common emergency in epileptology [2].
NCSE/NCS (non-convulsive seizure) was detected in 21% of 170 subjects in an intensive care unit. Clinical seizures preceded the EEG diagnosis of NCSE/NCS in only 25% of cases. The main risk factors for NCSE/NCS were previous CNS diseases, for example CNS tumors, known epilepsy, meningitis/encephalitis or evidence of encephalomalacia on MRI [12,13].
If the status epilepticus persists even after 1 hour or 24 hours of therapy, it is referred to as refractory or supra-refractory SE. Refractory and supra-refractory SE have a significantly worse outcome than uncomplicated status epilepticus. Strzelczyk et al. retrospectively investigated the outcome and duration of hospitalization of patients with refractory and super-refractory status epilepticus. For this purpose, the database “Gesundheitsforen Leipzig” was used, which contained inpatient and outpatient diagnoses, costs and demographic data for patients with an SE. The largest proportion of patients with non-refractory SE were discharged home (78.1%), whereas this applied to 70.1% of patients with RSE and only 31.7% of patients with SRSE. Over a third of patients with an SRSE (39.9%) died compared to 15% of RSE patients and 9.6% of nRSE patients [14].
New treatment approaches for the therapy of supra-refractory SE
New as well as already known treatment approaches are currently being discussed as possible treatment options for treatment-refractory status epilepticus. First and foremost, further attempts at drug therapy should be mentioned:
In a bicenter Swiss cohort study, 205 patients were included, 27% of whom were given anesthetics after the first-line drug failed. The results showed that anesthetics as second-line treatment is associated with shorter median duration of SE (0.5 versus 12.5 days, p<0.001), shorter ICU time (2 versus 5.5 days, p<0.001) and shortened hospital length of stay (8 versus 17 days, p<0.001) with equal rates of complications compared to anesthesia as third-line treatment [15].
Recently, the use of phenobarbital for the treatment of SRSE has been discussed. Phenobarbital is one of the oldest ASMs, having been in clinical use since 1912. There are various reports of a strong seizure-suppressive effect with little sedation. At the same time, possible side effects such as hypotension, arrhythmias, an increased infection rate and hypopnea have been reported during treatment with phenobarbital. Phenobarbital leads to an increase in GABAergic inhibition and to a reduction in glutamatergic excitation, as well as an inhibition of AMPA receptors. In individual cases, phenobarbital appears to be a therapeutic option for the treatment of RSE that should not be forgotten. As with other ASMs, an RCT would be desirable and necessary in order to assess the significance in the treatment of SE [16].
Non-drug treatment options discussed in the current guideline include the following: Transcranial direct current stimulation (tDCS), vagus nerve stimulator (VNS), ketogenic diet and focal cooling:
TDCS is a non-invasive neuromodulation technique that applies weak direct current stimulation across the scalp to induce linear and non-linear polarized effects. In particular, cathodic stimulation induces hyperpolarization in neuronal cells and provokes potentially relevant acute and long-term effects in the physiopathology of SRSE. Ng et al. tested the feasibility of using high-definition transcranial direct current stimulation (hd-tDCS) in the treatment of RSE. In 10 RSE patients, no adverse events occurred in 32 hd-tDCS sessions. TDCS could potentially be associated with an acute reduction in excitatory presynaptic input or depression of synaptic strength mediated by N-methyl-D-aspartate (NMDA) receptors, which may produce long-lasting effects including transmembrane action, protein migration and/or anti-inflammatory effects [17].
The treatment of supra-refractory SE with a ketogenic diet (KD) is a very promising approach. In a non-randomized retrospective cohort study, Koh et al. 140 RSE patients. This included 32 patients who were treated with a KD. Of these, the SE was broken at 28 (81%). The use of the KD influenced the reduction of the modified ranking scale (mRS) at discharge, in older patients, with higher seizure severity scores, under continuous intravenous anesthesia therapy (CIVAD) and in patients with SRSE. In addition, age and seizure severity scores, but not CIVAD or SRSE, were associated with KD-mediated change in mRS score at 3 months. Based on these data, the authors discuss a possible neuroprotective effect of KD in SRSE patients [18].
As a highly experimental method, Niesvizky-Kogan et al. recently introduced the principle of focal cooling as a treatment option for refractory epilepsies and SE. During cooling, there should be a reduction in the release of neurotransmitters from the presynapse and a loss of dendritic spines in the postsynapse. Furthermore, the electrical properties, nucleic acids, neurotransmitters, and cell membrane channel function are to be influenced. This is analogous to global cooling discussed in the context of neuroprotection in post-cardiovascular arrest and neonatal ischemic injury, but is discussed as a safer method [19].
New onset refractory status epilepticus (NORSE) poses a challenge for therapy. The majority of patients with new-onset refractory status epilepticus develop SRSE with a clinically unfavorable course and a mortality rate of 12-27% [3]. As Sculier et al. As described in a review, treatment is often difficult, with 75% of NORSE patients requiring anesthetic therapy. The authors were able to show that autoimmune encephalitis is the cause of a good half of all cases, so that early immunosuppressive therapy is recommended, such as prednisolone or, if necessary, intravenous immunoglobulins and plasma separation or, as second-line therapy, immunomodulatory drugs such as rituximab [3]. Patients with NORSE should also initially receive guideline-compliant therapy for SE. Should a refractory course of the newly occurring SE develop, Sculier et al. immunotherapy within the first 72 hours.
Another exciting therapeutic approach is the modulation of factors that influence status epilepticus, such as glucose metabolism and pyridoxal phosphate levels. In a monocentric retrospective cohort study by Müller et al. The study investigated whether the complications of intravenous treatment with valproate, which is used to treat SE, are different in patients with or without diabetes. During the study period, 408 patients and 482 episodes of SE were treated intravenously with VPA. Group comparisons showed no significant difference in treatment discontinuation rates. Differences were found in the rate of thrombocytopenia (p=0.015), which occurred more frequently in patients with diabetes. A total of 36 hypoglycemic episodes were identified, two occurred spontaneously under VPA. The authors concluded that diabetes as a relevant comorbidity carries a potentially increased risk of poor outcome after SE [20].
A retrospective cohort study by Rubinos et al. with a total of 293 patients investigated the relationship between pyridoxal phosphate (PLP) levels and established SE (eSE). The median PLP level of the eSE group (12 nmol/l) was lower than that of the ICU-noSE group (22 nmol/l, p=0.003), out-of-ICU (16 nmol/l, p=0.05) and outpatient groups (36 nmol/l, p <0.001). Patients with eSE thus had a significantly higher prevalence of marginal and decreased PLP levels (90 and 80%, respectively) compared to other ICU and non-ICU patients (ICU-noSE: 70, 50%; non-ICU: 63, 54%; outpatient: 38, 21%) [21]. However, therapeutic studies on the administration of PLP are still lacking.
Conclusion
Status epilepticus is one of the most common emergencies in neurology. The progression of refractory and super-refractory status epilepticus in particular continues to pose a challenge for clinical practice. A sufficiently high dose of initial therapy administered as soon as possible after diagnosis can reduce the rate of refractory courses. Status epilepticus, especially if consciousness is not maintained, is a potentially fatal condition that initially requires intensive medical treatment and monitoring. Therapy in line with the guidelines should be provided from the outset. New therapeutic approaches to treating RSE, such as the ketogenic diet or tDCS, which have shown promising initial results, should be considered. Further studies are needed here to assess the effectiveness outside of individual cases.
Take-Home-Messages
- Status epilepticus is defined as an epileptic seizure lasting >5 min or a series of seizures between which the original neurological status is not regained.
- One of the most important options for the primary treatment of status epilepticus is the rapid and adequate administration of benzodiazepines, which are available in various forms (intravenous, intramuscular, intranasal, buccal/sublingual, rectal).
- Status epilepticus should be treated in an intensive care unit.
Literature:
- Knake S, Rosenow F, Vescovi M, et al: Status Epilepticus Study Group Hessen (SESGH). Incidence of status epilepticus in adults in Germany: a prospective, population-based study. Epilepsia. 2001 Jun; 42(6): 714-718. doi: 10.1046/j.1528-1157.2001.01101.x. PMID: 11422324.
- Leitinger M; Trinka E; Giovannini G, et al: Epidemiology of status epilepticus in adults: A population-based study on incidence, causes, and outcomes (2019). In: Epilepsia 60(1), 53-62. doi: 10.1111/epi.14607.
- Sculier C, Gaínza-Lein M, Sánchez Fernández I, Loddenkemper T: Long-term outcomes of status epilepticus: A critical assessment (2018). In: Epilepsia 59 Suppl 2, 155-169. doi 10.1111/epi.14515.
- Rosenow F, Weber J, et al: Status epilepticus in adults. S2k guideline. (2020): Ed. by the German Society of Neurology (DGN). Available online at www.dgn.org/leitlinien, last updated on 30.06.2020, last reviewed on 27.10.2023.
- Misra Usha K, Dubey D, Kalita J: A randomized controlled trial of lacosamide versus sodium valproate in status epilepticus (2017). In: Epilepsia. doi: 10.1111/epi.13706.
- Strzelczyk A, Steinig I, Willems LM, et al: Treatment of refractory and super-refractory status epilepticus with brivaracetam: A cohort study from two German university hospitals. (2017b). In: Epilepsy & behavior: E&B 70 (Pt A), 177-181. doi: 10.1016/j.yebeh.2017.03.028.
- Guterman EL, Burke JF, Sporer KA: Prehospital Treatment of Status Epilepticus in the United States (2021). In: JAMA 326 (19), 1970-1971. doi: 10.1001/jama.2021.15964.
- Kellinghaus C, Rossetti AO, Trinka E, et al: SENSE registry for status epilepticus. Epilepsia. 2018 Oct; 59 Suppl 2: 150-154.
doi: 10.1111/epi.14495. Epub 2018 Aug 29. PMID: 30159884 - Halliday AJ, Santamaria J, D’Souza WJ: Pre-hospital benzodiazepines associated with improved outcomes in out-of-hospital status epilepticus: A 10-year retrospective cohort study (2021). In: Epilepsy research 179, 106846. doi: 10.1016/j.eplepsyres.2021.106846.
- Gawedzki P, Celmins L, Fischer D: Pharmacist involvement with antiepileptic therapy for status epilepticus in the emergency department (2022). In: The American journal of emergency medicine 59, 129-132. doi: 10.1016/j.ajem.2022.07.002.
- Drislane FW: Presentation, evaluation, and treatment of nonconvulsive status epilepticus (2000). In: Epilepsy & behavior: E&B 1 (5), 301-314. doi: 10.1006/ebeh.2000.0100.
- Laccheo I, Sonmezturk H, Bhatt AB, et al: Non-convulsive status epilepticus and non-convulsive seizures in neurological ICU patients (2015). In: Neurocritical care 22 (2), 202-211. doi: 10.1007/s12028-014-0070-0.
- Spindler M, Jacks LM, Chen X, et al: Spectrum of nonconvulsive status epilepticus in patients with cancer (2013). In: Journal of clinical neurophysiology: official publication of the American Electroencephalographic Society 30 (4), 339-343. doi: 10.1097/WNP.0b013e31829ddcdb.
- Strzelczyk A, Ansorge S, Hapfelmeier J, et al: Costs, length of stay, and mortality of super-refractory status epilepticus: A population-based study from Germany (2017a). In: Epilepsia 58 (9), 1533-1541. DOI: 10.1111/epi.13837.
- Sutter R, Jünger AL, Baumann SM, et al: Balancing the risks and benefits of anesthetics in status epilepticus (2023). In: Epilepsy & behavior: E&B 138, 109027. doi: 10.1016/j.yebeh.2022.109027.
- Trinka E: Phenobarbital in status epilepticus – Rediscovery of an effective drug. Epilepsy Behav 2023 Apr; 141: 109104. doi: 10.1016/j.yebeh.2023.109104.
- Ng MC, El-Alawi H, Toutant D, et al: A Pilot Study of High-Definition Transcranial Direct Current Stimulation in Refractory Status Epilepticus: The SURESTEP Trial (2023). In: Neurotherapeutics: the journal of the American Society for Experimental NeuroTherapeutics 20(1), 181-194. doi: 10.1007/s13311-022-01317-5.
- Koh S, Kim T-J, Shin H-B, et al.: Expanding Indications for a Ketogenic Diet as an Adjuvant Therapy in Adult Refractory Status Epilepticus: an Exploratory Study Using Moderation Analysis (2022). In: Neurotherapeutics: the journal of the American Society for Experimental NeuroTherapeutics 19 (5), 1526–1534. doi: 10.1007/s13311-022-01282-z.
- Niesvizky-Kogan I, Bass M, Goldenholz SR, Goldenholz M: Focal Cooling for Drug-Resistant Epilepsy: A Review (2022). In: JAMA neurology 79 (9), 937-944. doi: 10.1001/jamaneurol.2022.1936.
- Müller A, Hofen-Hohloch Jv, Awissus C, et al: Does diabetes mellitus affect the safety profile of valproic acid for the treatment of status epilepticus? A retrospective cohort study (2022). In: Neurological research and practice 4(1), 52. doi: 10.1186/s42466-022-00212-w.
- Rubinos C, Bruzzone MJ, Blodgett C, et al: Association of Serum Pyridoxal Phosphate Levels with Established Status Epilepticus (2023). In: Neurocritical care 38(1), 41-51. DOI: 10.1007/s12028-022-01579-z.
HAUSARZT PRAXIS 2024; 19(9): 12–16