The diagnosis of COPD is often made at an advanced stage. Earlier diagnosis, which would lead to a significant improvement in prognosis, requires screening spirometrics in the primary care physician’s office in all smokers, e.g., at age 40 years. Awareness must be raised that the diagnosis of COPD is based exclusively on spirometric criteria [1, 2] (FEV1/FVC <70% after bronchospasmolysis)! In the following article, the therapeutic aspects of COPD are discussed.
A causally oriented therapy of COPD takes into account which COPD phenotype [3] is present: COPD with emphysema (“pink puffer,” “blue bloater”), with frequent exacerbations, with excessive annual FEV1 loss, and with an asthma component. Specific clinical problems arise from these phenotypes that require targeted therapy.
Emphysema: Structural and dynamic lung hyperinflation
COPD patients with emphysema often have dynamic hyperinflation in addition to structural hyperinflation, which occurs with forced breathing during physical activity. In addition to the factors of bronchospasm, mucosal swelling, and subendothelial fibrosis, emphysema with loss of elasticity of the lungs can lead to collapse of the bronchi during forced expiration (Fig. 1). Tethered air forms with a consecutive reduction in vital capacity, resulting in a transient increase in exertional dyspnea [4].
Two strategies reduce dynamic hyperinflation: first, the use of the lip brake during physical activity [5]. However, the one-time instruction of the lip brake is not enough! Within the framework of training therapy, the therapist must repeatedly check whether the patient is also automatically implementing what he or she has learned in everyday life. Second, the use of long-acting bronchodilators (inhaled beta stimulants and anticholinergics). With peripheral bronchodilation, dynamic lung hyperinflation decreases; this is referred to as “pharmacologic volume reduction.”
COPD with excessive annual FEV1 loss.
Emphysema patients have an even greater annual FEV1 loss of >60 ml compared to other COPD phenotypes. If this occurs at a young age or with only low nicotine use, serum α-1-antitrypsin levels should be determined. Recently, substitution therapy has been covered by health insurance. However, the indication must be made by the pulmonologist, which is why a pneumological consultation is advisable when COPD is diagnosed.

COPD with frequent acute exacerbations
Patients with frequent severe COPD exacerbations show a rapid decline in their clinical condition and a rapid increase in mortality [6]. Triggers of acute exacerbations include viral respiratory infections, primary bacterial exacerbations, massive exposure to particulate matter, and forced reduction in physical activity, which can lead to secretory stasis in the bronchi. Bronchial secretions are breeding grounds for pathogenic germs. In the presence of secretory stasis (bronchiectasis, severe obstruction), the risk of bacterial colonization, including with Pseudomonas, also increases, further worsening the prognosis.
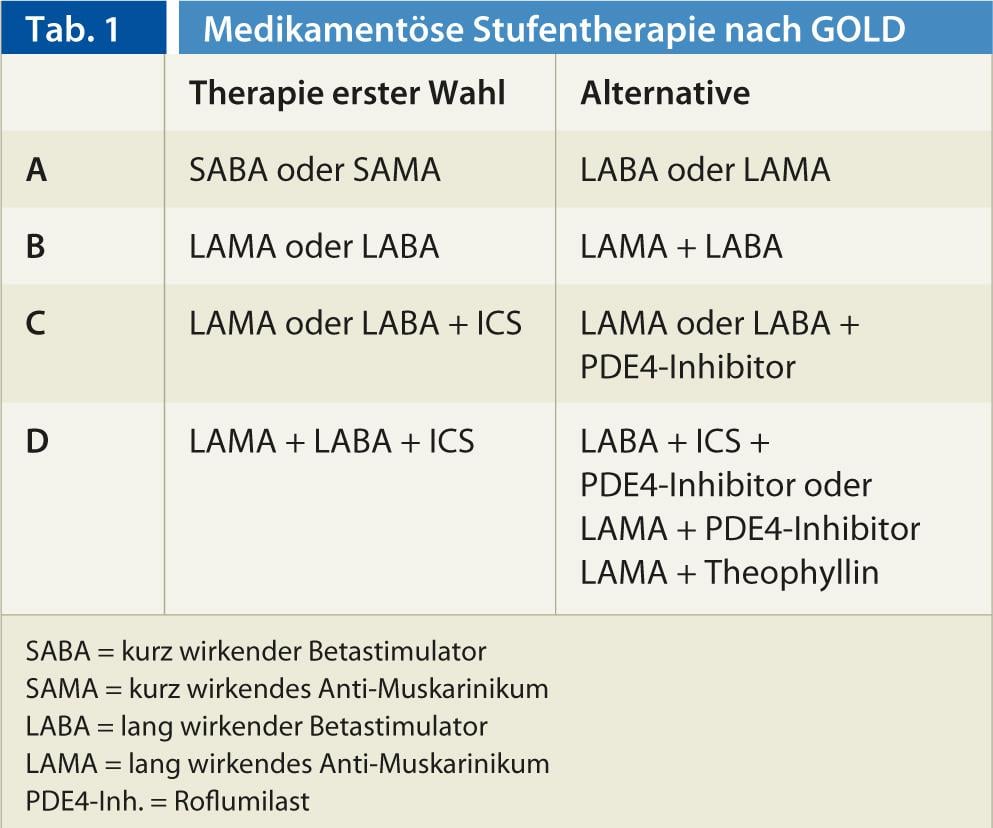
What can be done to reduce the frequency of acute exacerbations? Sufficient physical activity contributes to this. Except in asymptomatic cases, primary outpatient pulmonary rehabilitation is recommended very early in COPD, including targeted physical training. Annual flu shots and pneumococcal vaccination are also essential. Antibiotic therapy of an exacerbation with increased and purulent sputum prolongs the time to the next exacerbation [7]. Several drugs such as long-acting beta stimulants, long-acting anticholinergics, N-acetylcysteine, oral bacterial lysates, topical (inhaled) corticosteroids, and the new PDE-4 inhibitor Roflumilast (Daxas®) have been shown to reduce exacerbations. Roflumilast and inhaled topical steroids are indicated only in advanced COPD with frequent exacerbations. In general, the intensity of baseline drug therapy correlates with GOLD stages. The therapy levels of the current GOLD classification [1] can be found in Table 1.
COPD with asthma component
A rare variant of high-eosinophil adult-onset asthma does not exhibit bronchial hyperreactivity, i.e., acute asthma attacks. Instead, there is slowly progressive mucosal swelling with consecutive exertional dyspnea, which is potentially reversible under high-dose steroids. This form of asthma may mimic COPD, as bronchospasmolysis testing often shows no improvement [8].
More often, over the years, a proportion of fixed bronchial obstruction develops in asthma as well (“airway remodeling”), so that formally the spirometric criteria of COPD are met. However, these patients still have active asthma clinically and anamnestically and must be treated accordingly as asthma patients.
It is not uncommon to detect an asthma component in COPD patients. The medical history sometimes shows asthma in childhood, which then “grew out” during puberty. Later came the nicotine abuse. Affected individuals often exhibit partial reversibility of obstruction, i.e., FEV1 improves with bronchospasmolysis by >12% and >200 ml absolute. These patients partially benefit from therapy with topical steroids, even if the COPD is not yet severe and rarely has severe exacerbations.
Other therapeutic strategies in COPD
Therapy of exacerbation: Exacerbations can be mild, i.e. improve spontaneously or by increased inhalation. Moderate-severe exacerbations require a short steroid burst and/or antibiosis. Exacerbations are classified as severe when emergency hospitalization is required.
Today, the recognition of an exacerbation is still based on the 1986 Anthonisen criteria. These are: Increase in dyspnea, sputum, and new onset sputum purulence. Even if two criteria are present, intervention should occur. If increased sputum and purulence dominate, an antibiotic is useful, e.g., five to seven days of amoxicyllin/clavulanic acid. If there is a massive increase in dyspnea, short burst therapy with an oral corticosteroid is indicated, e.g., 30 mg prednisone for five days. A longer duration of therapy does not bring more [9]. However, except for short bursts of steroids during exacerbations, oral steroids should be avoided in the continuous therapy of COPD, as they promote osteoporosis and, because of their catabolic effect on muscle, have the opposite effect of what pulmonary rehabilitation is intended to achieve.
Reduction of exertional dyspnea: use of lip-blocking and long-acting bronchodilators reduce exertional dyspnea. In addition, physical activation represents an important therapeutic strategy. People with COPD try to avoid the agonizing shortness of breath on exertion by taking it easy. Consecutively, muscle atrophy occurs. The deconditioned muscle now requires more oxygen for the same performance, i.e. after an inactive phase even a previously still tolerated performance level already leads to respiratory distress. Thus, even acute hospitalization for pneumonia, as well as for a lower extremity fracture, can exacerbate exertional dyspnea. Pulmonary rehabilitation [10] attempts to counteract this downward spiral. This requires intensive endurance and strength training, which must be monitored by specialists due to dyspnea and frequent polymorbidity. Physical activity improves patient prognosis and reduces mortality [11].
Secretion drainage: patients with abundant bronchial secretions depend on regular secretion drainage, especially because COPD-related epithelial metaplasia impairs mucociliary clearance. Cough clearance alone is insufficient. In addition, decades-long coughing can damage the wall structure of the bronchi, causing coughing collapse of the central bronchi with corresponding distal secretory stasis. A good “bronchial toilet” includes drinking plenty of fluids in the morning, inhaling with a beta stimulator, breathing with a PEP-device (PEP = positive expiratory pressure), e.g. a bubble bottle, the Flutter®, Cornet® or Acapella®. A short period of physical activity, such as on an exercise bike, also helps to mobilize secretions by speeding up the flow of breath and then making them easier to expel.
COPD self-management: analogous to diabetes patients, a COPD patient should acquire knowledge about their disease with the aim of avoiding noxious agents, living actively, inhaling correctly, and assessing their health condition correctly so that they can recognize an exacerbation early and respond adequately if they are away from home. Within the framework of the certified pulmonary rehabilitation programs in Switzerland [12], the necessary knowledge is imparted. For this purpose, a very good teaching aid exists from “Lunge Zürich”, which will also be available from all cantonal lung leagues in the future. The brochure is called “Living Better with COPD.
Nicotine stop: Nicotine stop still represents the only means to effectively reduce excessive annual FEV1 loss. The patient’s willingness to stop smoking must be induced by frequently discussing smoking in a way that is tolerable for the patient. Once this is available, concrete proposals will make sense. Various drug strategies exist for this, including vareniclinum (Champix®) or nicotine replacement products. Dispensing a patch alone often proves insufficient, as the ultimate craving for a cigarette can still arise. Accordingly, the addition of a fast-acting nicotine substitute (Nicorette® chewing gum or inhaler) is useful. Many lung leagues offer good smoking cessation classes that can be extremely helpful for patients.
Oxygen therapy: in COPD with emphysema, ventilatory and diffusion impairment do not always correlate well, i.e., even with only moderate obstruction, significant partial respiratory insufficiency (pO2 <55 mgHg, SaO2 <90%) may already be present in the exacerbation-free interval, especially if patients live in the mountains. Accordingly, screening by spot pulse oximetry and appropriate referral to a pulmonologist are important.
Long-term home oxygen therapy with concentrator or liquid oxygen (LOX) is not primarily intended to reduce respiratory distress, but to prevent cor pulmonale. However, it must be applied at least 16 out of 24 hours per day for the desired effect to occur. For vacation incl. Air travel there are now also mobile concentrators, which are rented by many lung leagues for this purpose.
Volume reduction: In very advanced COPD, the potential for improvement is often limited despite exhaustion of all conservative therapeutic options. Therefore, in younger patients, lung transplantation must be considered. In older ones, thoracoscopic volume reduction is sometimes considered. Only limited experience is available with regard to bronchoscopic volume reduction procedures.
With the removal of parts of the lungs that are practically worthless for oxygenation, the diaphragms are raised, diaphragmatic breathing is improved again. The overinflated lungs not only expanded outward, but also compressed the bronchi located in the lungs, increasing obstruction and work of breathing. With volume reduction, this situation improves and there is a slight improvement in pulmonary function, but usually an impressive improvement in the 6-minute walk test.
Conclusion
Therapeutic nihilism is no longer appropriate in COPD today. Therapeutic options have increased significantly in recent years. Regaining physical activity is of central importance in this context. Nevertheless, primary prophylaxis and early detection of COPD must be a primary focus in practice as well.
CONCLUSION FOR PRACTICE
- The diagnosis of COPD is based exclusively on spirometric criteria.
- There are four COPD phenotypes: COPD with emphysema (“pink puffer,” “blue bloater”), with frequent exacerbations, with excessive annual FEV1 loss, and with an asthma component.
- In general, the intensity of baseline drug therapy correlates with GOLD stages.
- It is not uncommon to detect an asthma component in COPD patients.
- Regaining physical activity is of central importance in COPD therapy.
Thomas Rothe, MD
Literature:
- www. goldcopd.org
- Steurer-Stey C, et al: COPD – Quintessence for the primary care provider. Schweiz Med Forum 2013; 13: 227-230.
- Weatherall M, et al: Distinct clinical phenotypes of airways disease defined by cluster analysis. Eur Respir Dis 2009; 34: 812-818.
- Rothe T: Spirometry for clinical practice. PRAXIS 2012; 101: 1481-1487.
- Visser FJ, et al: Pursed-lips breathing improves inspiratory capacity in COPD. Respiration 2011; 81: 372-378.
- Suissa S, et al: Long-term natural history of COPD: Severe exacerbations and mortality. Thorax 2012; 67: 957-963.
- Llor C, et al: Efficacy of antibiotic therapy for acute exacerbations of mild to moderate COPD. Am J Respir Crit Care Dis 2012; 186: 716-723.
- Rothe T: COPD and asthma: Same, same but different. PRAXIS 2012; 101: 233-237.
- Leuppi JD, et al: Short-term versus conventional glucocorticoid therapy in acute exacerbations of chronic obstructive pulmonary disease: the REDUCE randomized clinical trial. JAMA 2013; 309: 2223-2231.
- Puhan M, et al: Pulmonary rehabilitation – A modern and patient-oriented therapeutic approach in COPD. PRAXIS 2013; 102: 99-106.
- Waschki B, et al: Physical activity is the strongest predictor of all-cause mortality in patients with copd. Chest 2011; 140: 331-342.
- www.pneumo.ch/de/informationen-fuer-fachpersonen/pulmonale-rehabilitation/anerkannte-zentren.html.