The monoclonal antibodies casirivimab/imdevimab and sotrovimab received Swissmedic approval last December and this January, respectively. Another promising drug for the treatment of Covid 19 patients is expected to be launched in Switzerland soon. Molnupiravir is an orally administered antiviral that should be used in early-stage covid 19 symptomatology, recent trial data show.
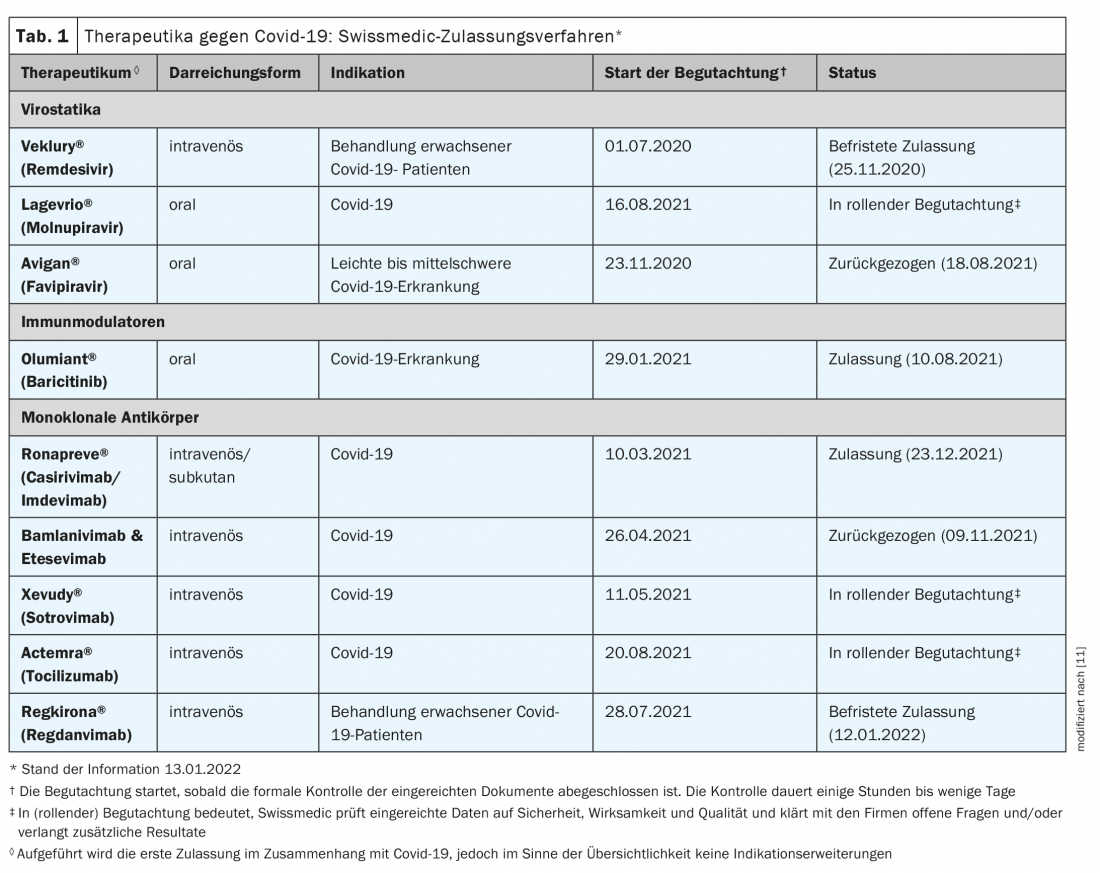
Vaccination is the most important tool to protect against infections, severe courses, and hospitalizations [1]. However, for those who do become infected, safe and effective covid-19 therapeutics are critical. Promising drug candidates contribute in particular to a reduction in the need for hospitalization and counteract severe courses of hospitalization. According to appropriate reviews, the following three drug categories meet these criteria: antiviral monoclonal antibodies, antiviral oral agents, and immunomodulators [1]. While the former are designed to prevent viruses from entering or replicating in body cells, immunomodulators are used in the advanced stages of the disease to dampen the body’s defense responses [2]. The FOPH regularly exchanges information with the scientific task force on existing and new therapies against covid-19. The marketing authorization applications currently under review at Swissmedic are shown in Table 1 [3,15].
First oral drug for the treatment of outpatients
The Federal Office of Public Health has contracted for the reservation of the antiviral drug molnupiravir (Lagevrio®) on the recommendation of the Covid 19 Scientific Task Force. Available in tablet form, molnupiravir is an RNA polymerase inhibitor and is one of the antiviral agents. The drug has already been approved in the UK since November 2021, and an emergency approval was filed in the US during the same period [2]. A clinical trial has demonstrated efficacy in non-hospitalized covid-19 patients at high risk for severe disease progression, where the time window appears to be critical to treatment success [4,5] (Box). The drug can already be used in Switzerland during the current approval procedure after an adaptation of the Covid 19 Regulation 3, which still has to be made (status of information: Jan. 15, 2022). The federal government will cover the cost of treatment in the outpatient setting until it is reimbursed by mandatory health insurance [3].

Sotrovimab and casirivimab/imdevimab now officially approved
For Xevudy® (sotrovimab), official Swissmedic approval was granted on Jan. 14, 2022, and for Ronapreve® (casirivimab and imdevimab) on Dec. 23, 2021 [6]. Both are intravenous monoclonal antibodies indicated for adults and adolescents 12 years of age and older (body weight at least 40 kg) for the treatment of covid-19 when oxygen therapy or hospitalization is not required and there is a high risk of developing a severe covid-19 course. Ronapreve® is also indicated – as the only drug approved in Switzerland to date – for the prevention of Covid-19 when an adequate immune response to Covid-19 vaccination is not possible due to other diseases or therapies. However, no information on efficacy in the omicron variant was available at the time of approval [6].
Remdesivir – What’s new?
Remdesivir (Veklury®) has already been available in Switzerland for the treatment of Covid-19 since 2020. There was and still is a controversial debate about the benefits of the active ingredient, which was originally developed to treat Ebola viruses. From a December 2021 publication of new trial data in the New England Journal of Medicine, a three-day course of remdesivir is associated with a lower risk of hospitalization or death compared with placebo in nonhospitalized patients at high risk for progression to covid-19 [7]. The study included non-hospitalized patients with covid-19 who had symptoms within the previous seven days and had at least one risk factor for disease progression. Patients were randomly assigned to treatment with remdesivir (n=279) or placebo (n=283). Remdesivir was administered intravenously at a dose of 200 mg on day 1 and 100 mg on days 2 and 3. Covid-19-related hospitalizations or deaths occurred in two patients in the remdesivir group and in 15 in the placebo group (0.7% vs. 5.3%; hazard ratio: 0.13). By day 28, 1.6% and 8.3% of patients in the remdesivir and placebo groups, respectively, had a Covid 19-related physician visit (hazard ratio: 0.19). There were no deaths through day 28. Adverse events occurred in 42.3% and 46.3% of patients in the verum and placebo groups, respectively.
And what about Paxlovid®?
Used at an early stage of Covid disease, this orally administered drug can significantly attenuate the course of the disease, according to current evidence. In studies, Paxlovid® (nirmatrelvir plus ritonavir) reduced the risk of hospitalization and death by 89% in adult non-hospitalized high-risk Covid 19 patients when they were treated with it for five days within three days of symptom onset [8]. The U.S. Food and Drug Administration (FDA) has pronounced an emergency approval for Paxlovid® at the end of December 2021 for the indication of positive corona patients 12 years of age and older with mild to moderate symptoms and a high risk of a severe course [10]. The European Medicines Agency (EMA) supports the use of Paxlovid® for the treatment of adults with covid-19 who do not require supplemental oxygen and who are at increased risk of severe disease progression [9].
Literature:
- “Questions and Answers on the List of Ten COVID-19 Therapeutic Candidates,” 10/22/2021, https://ec.europa.eu (last accessed 01/15/2022).
- “Therapeutic Drugs for Coronavirus Infection Covid-19,” Jan. 11, 2022, www.vfa.de/de/arzneimittel-forschung (last accessed Jan. 15, 2022).
- “Coronavirus: federal government signs contract to reserve drug,” www.bag.admin.ch, (last accessed Jan. 15, 2022).
- Jayk Bernal A, et al; MOVe-OUT Study Group. Molnupiravir for Oral Treatment of Covid-19 in Nonhospitalized Patients. N Engl J Med. 2021 Dec 16: NEJMoa2116044.
- “Phase 3 study confirms efficacy of molnupiravir in non-hospitalized COVID-19 sufferers,” German Society of Neurology, Jan. 07, 2022, https://dgn.org/neuronews (last accessed Jan. 15, 2022).
- “Swissmedic Approves “Ronapreve(R)” for Covid 19 Patients” , Dec. 27, 2021, www.swissmedic.ch (last accessed Jan. 15, 2022)
- Gottlieb RL, et al; GS-US-540-9012 (PINETREE) Investigators. Early Remdesivir to Prevent Progression to Severe Covid-19 in Outpatients. N Engl J Med. 2021 Dec 22. doi: 10.1056/NEJMoa2116846.
- “One Million Packs of Pfizer’s Covid 19 Drug: Federal Government Buys Paxlovid,” www.deutsche-apotheker-zeitung.de (last accessed Jan. 15, 2022).
- “EMA Supports Use of Paxlovid for Emergencies,” www.aerzteblatt.de/, (last accessed Jan. 15, 2022).
- “Pfizer Receives U.S. FDA Emergency Use Authorization for Novel COVID-19 Oral Antiviral Treatment,” www.pfizer.com/news (last accessed Jan. 15, 2022).
- “Status of Approvals for Covid-19 Control,” www.swissmedic.ch (last accessed Jan. 15, 2022).
- “BIO COVID-19 Therapeutic Development Tracker,” www.bio.org (last accessed Jan. 15, 2022).
- Whitley R: Molnupiravir – A Step toward Orally Bioavailable Therapies for Covid-19. N Engl J Med. 2021 Dec 16: NEJMe2117814.
HAUSARZT PRAXIS 2022; 17(1): 40-41
InFo PNEUMOLOGY & ALLERGOLOGY 2022; 4(1): 34-35.
CARDIOVASC 2022; 21(1): 34-35












