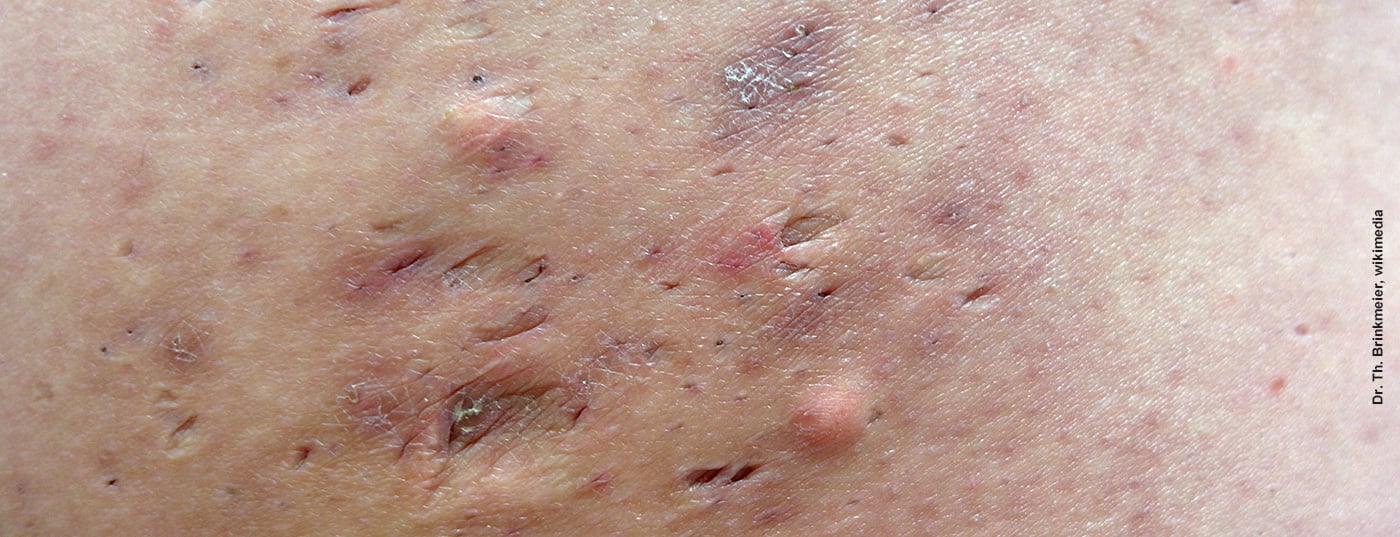
Patients affected by acne inversa often have a high level of suffering due to the painful abscesses and fistulas. In addition to impaired quality of life, incapacity to work and social withdrawal can be the result. While there is no cure for acne inversa, there are several treatment options available to help curb symptoms. In order to evaluate the course of therapy, “patient-reported outcomes” have gained importance, in addition to instruments for objective measurement of severity.
Compared to other dermatological diseases, acne inversa (also called “hidradenitis suppurativa”) is one of the most distressing diseases ever [1]. This chronic inflammatory recurrent skin disease is manifested by repeated abscesses, mainly in the axillary, pubic and inguinal areas, but also in other skin folds. The pus collections are accompanied by swollen, inflammatory reddened tissue and are very painful [2]. Most often, the disease occurs in early adulthood [3]. In the pathogenesis, a cornification disorder of the sebaceous glands at the hair roots plays an important role [4]. This causes hyperkeratosis, which leads to occlusion of the excretory canal, and as a result, the sebaceous gland and hair root become inflamed. Invading bacteria multiply, pus accumulates and an abscess develops. Adjacent sweat glands may also be affected. Starting from an abscess, a fistula may develop, with a pathological connection to the skin surface. Secretion then flows out through this duct, which can lead to oozing and an unpleasant odor. Scars often form on the affected areas of skin.
Older and newer severity classification instruments
Objective assessment of severity is a prerequisite for determining the appropriate therapeutic strategy. Hurley classification is the oldest and most widely used methodology. It is a static classification into stages: stage I (single abscesses without fistula formation), stage II (recurrent abscesses with fistula formation and scarring cords), and stage III (diffuse involvement with abscesses, fistula ducts, and scarring) [5]. The modified Sartorius assessment provides a dynamic assessment of disease severity [6]. In the Sartorius score, the number of inflammatory nodules, abscesses, fistulas, and affected areas are recorded and assigned points. Both the Hurley classification and the Sartorius score are relatively complex and of limited use in everyday practice. In a consensus process of members of the European Hidradenitis Suppurativa Foundation (EHSF), another score was developed several years ago, the International Hidradenitis Suppurativa Severity Score System (IHS4) [7]. The IHS4 score allows a dynamic and individual classification of the disease. All inflamed lesions are scored and summed, with an inflamed nodule counting single, an abscess counting double, and a draining fistula scoring quadruple. The sum of the individual assessed lesions results in a classification into three severity levels (mild, moderate, severe) [7]. The Physician Global Assessment (PGA) is also frequently used in clinical trials [8,9].
Acne inversa is complicated and multiform, but treatable
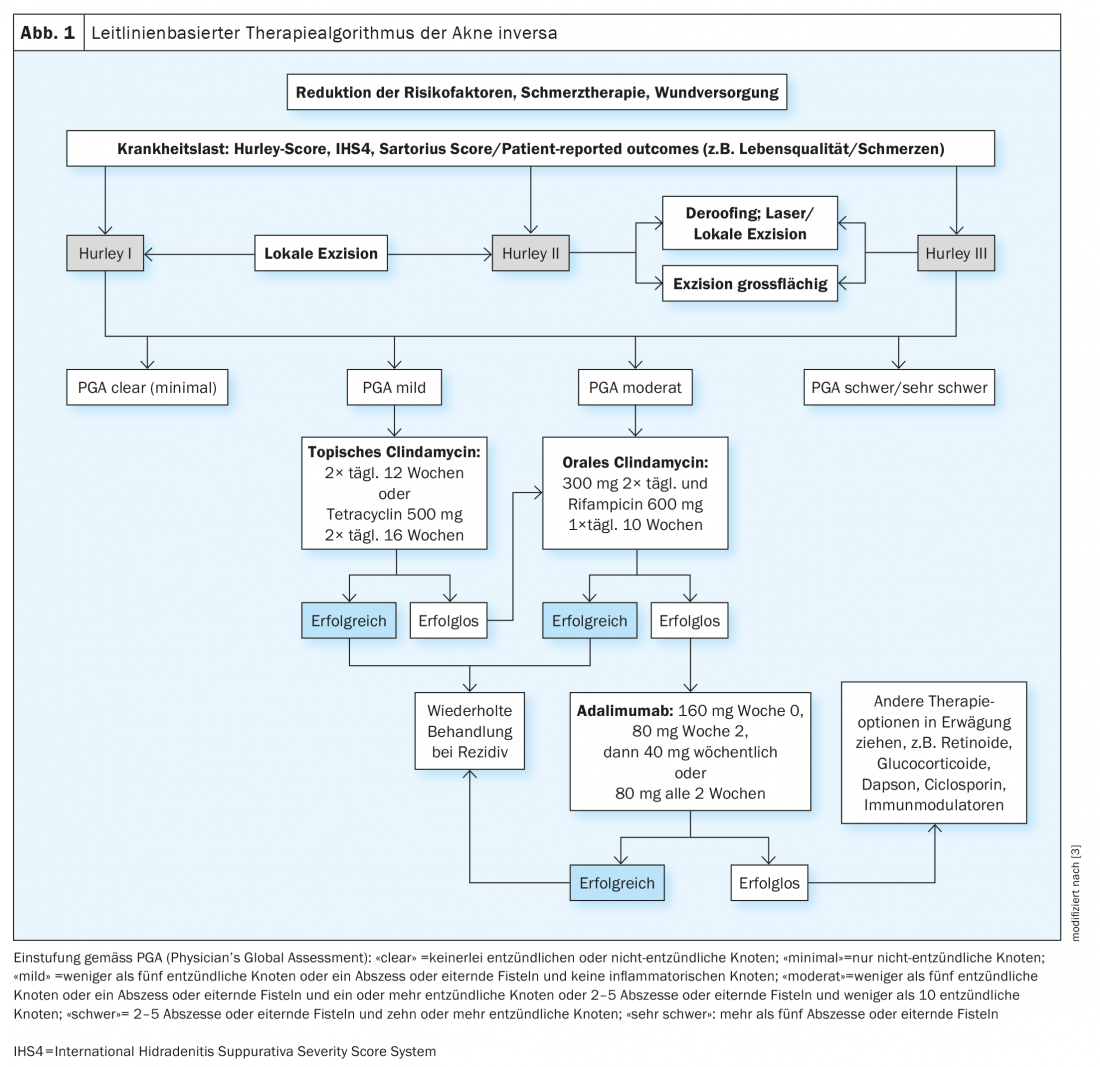
Based on the European guidelines, Gulliver et al. developed a treatment algorithm. This is based on the treatment recommendations contained in the guidelines, which were assessed using the Grading of Recommendations Assessment and Evaluation (GRADE) method (Fig. 1) [3,8,10]. Various measures are often combined to bring acne inversa under control [2]. For milder to moderate forms of the condition, physical therapy using a specific combination of light (Intense pulsed light, IPL) and radiofrequency can be effective. Surgical rehabilitation is still considered the gold standard, especially for localized forms of disease. However, patients often have to put up with weeks or months of downtime until the wound has healed. In addition, relapses or the appearance of abscesses in other localizations may occur. In terms of drug treatment options, antibiotics are essential for derailing infections. However, antibiotics are not suitable as long-term therapy because of the risk of side effects and because they can promote the development of resistance. For the treatment of moderate to severe acne inversa, the monoclonal IgG1 antibody adalimumab is currently the only biologic approved. For adult patients who do not respond to or cannot tolerate systemic antibiotics, adalimumab is recommended as first-line treatment [11]. Adalimumab should be administered at a dose of 160 mg at week 0, 80 mg at week 2, and 40 mg per week starting at week 4. If no response to therapy is achieved after 16 weeks, continuation of treatment should be reconsidered.
Progress measurement with inclusion of subjective patient assessment
The guideline lists the treatment goals as improvement of the disease by one severity level according to Hurley’s classification and/or improvement of the Sartorius score or the Dermatology Life Quality Index (DLQI) by 25% within 12 weeks. [12]. The recording of patients’ subjective assessment of treatment goals and benefits using patient-reported outcome measures (PROMs) plays an increasingly important role [13]. Systematically collected PROMs, combined with evidence-based information, can help align medical care with patient needs, values, and preferences [14]. The use of PROM before, during, and/or after treatment demonstrates changes at the individual patient level, such as improved physical functioning or quality of life [15].
The European Academy of Dermatology and Venereology Task Force on Quality of Life and Patient-Centered Outcomes suggests the use of the Dermatology Life Quality Index (DLQI) for patients with acne inversa, among others, but also advocates the use and further development of other validated acne inversa-specific PROMs [1].
Acquisition of the acne inversa-specific quality of life
In recent years, several efforts have been made to develop patient-reported outcome measures that map acne inversa-specific quality of life changes. Thorlacius et al. have published pilot studies on this in 2019, and Kirby et al. published a 17-item HiSQoL score (The Hidradenitis Suppurativa Quality of Life score) in 2020 [16]. It is a questionnaire that contains more general skin-related items as well as acne inversa-specific items such as discharge and odor [1]. In convergent validity analyses, a high correlation was found between the HiSQoL and the DLQI [17]. The HiSQoL was developed primarily for use in clinical trials, but there are plans to develop a scaled-down version of the questionnaire for use in everyday clinical practice, as described in the 2020 publication by Kirby et al. to be taken from [16].
Early vs. late onset of disease
Acne inversa usually appears in early adulthood, but may begin later in life. Recently, a threshold of 28 years was proposed to distinguish between patients with early and late onset. To compare the quality of life and psychological distress of sufferers with early vs. late onset, a cross-sectional study examined 467 over 16-year-old patients diagnosed with acne inversa [19]. Patients were categorized as “early onset” (<28 years) and “late onset” (≥ 28 years). The average age at the time of diagnosis was 21.6 years. The 22.0% of patients who had late disease onset reported poorer psychosocial quality of life and higher distress than those with early disease onset. No differences were observed between the two groups in terms of clinical severity. According to multivariate analyses, the following factors were significantly associated with a later onset: higher number of fistulas, higher BMI, ex-smoking status, no manifestations in the axillae, no manifestations in the breast region, the presence of psoriasis, and higher scores on the psychosocial scale of the Skindex-17. In conclusion, the results of this study indicate that the psychosocial impact is greater in patients with a late onset of the disease.
Literature:
- Zouboulis CC, Chernyshov PV: Hidradenitis suppurativa-specific, patient-reported outcome measures. J Eur Acad Dermatol Venereol 2021; 35(7): 1420-1421.
- “Acne inversa – protracted but treatable,” 05/2019, www.bvdd.de/aktuelles-presse, (last accessed Sept. 22, 2022).
- Gulliver W, et al: Evidence-based approach to the treatment of hidradenitis suppurativa/acne inversa, based on the European guidelines for hidradenitis suppurativa. Rev Endocr Metab Disord 2016; 17(3): 343-351.
- Lowe MM, et al: Immunopathogenesis of hidradenitis suppurativa and response to anti-TNF-α therapy. JCI Insight 2020; 5(19): e139932.
- Hurley HJ: Axillary hyperhidrosis, apocrine bromhidrosis, hidradenitis suppurativa and familial benign pemphigus. Surgical approach. In: Dermatologic Surgery. Principles and Practice (Roenigk RK, Roenigk HH Jr, eds)2nd edn. New York, 1996: 623-645.
- Sartorius K, et al: Objective scoring of hidradenitis suppurativa reflecting the role of tobacco smoking and obesity. Br J Dermatol 2009; 161: 831-839.
- Zouboulis CC, et al; European Hidradenitis Suppurativa Foundation Investigator Group. Development and validation of the International Hidradenitis Suppurativa Severity Score System (IHS4), a novel dynamic scoring system to assess HS severity. Br J Dermatol 2017; 177(5): 1401-1409.
- Zouboulis CC, et al: European S1 guideline for the treatment of hidradenitis suppurativa/acne inversa. J Eur Acad Dermatol Venereol 2015; 29(4): 619-644.
- Kimball AB, et al: Adalimumab for the treatment of moderate to severe hidradenitis suppurativa: a parallel randomized trial. Ann Intern Med 2012; 157: 846-855.
- Guyatt G, et al: GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ April 2008; 336:924.
- Swiss Drug Compendium, https://compendium.ch, (last accessed Sept. 22, 2022).
- Zouboulis CC, et al: Therapy of hidradenitis suppurativa/acne inversa, AWMF Registry Number 013-012, www.awmf.org, (last accessed Sept. 22, 2022).
- Kirsten N, et al: HS-PBI: disease-specific questionnaire to assess patient-defined treatment goals and benefits for acne inversa. JDDG 2018; 16 (IssueS1), 2-30.
- Hostettler S, Kraft E, Bosshard C: Patient-reported outcome measures: The patient perspective counts. Schweiz Ärzteztg 2018; 99(40): 1348-1352.
- Ovretveit J, et al: Using patient-reported outcome measurement to improve patient care. Int J Qual Health Care 2017; 29(6): 874-879.
- Kirby JS, et al: The Hidradenitis Suppurativa Quality of Life (HiSQOL) score: development and validation of a measure for clinical trials. Br J Dermatol 2020; 183(2): 340-348.
- Kursawe Larsen C, et al: Convergent validity of suffering and quality of life as measured by the Hidradenitis Suppurativa Quality of Life. J Eur Acad Dermatol Venereol 2021; 35: 1577-1581.
- Tzellos T, Zouboulis CC: Which hidradenitis suppurativa comorbidities should I take into account? Exp Dermatol 2022; 31 (Suppl 1): 29-32.
- ampogna F, et al: Quality of life in patients with early- and late-onset hidradenitis suppurativa. Arch Dermatol Res 2022 Aug 23. doi: 10.1007/s00403-022-02374-8.
- Thorlacius L, et al: Development of HiSQOL: A Hidradenitis Suppurativa-Specific Quality of Life Instrument. Skin Appendage Disord 2019; 5(4): 221-229.
DERMATOLOGIE PRAXIS 2022; 32(5): 35-38