With early diagnosis and treatment, the life expectancy of HIV-infected individuals approaches that of otherwise health comparable HIV-negative individuals. According to the FOPH guidelines, HIV testing should be performed in a variety of clinical indicator situations to allow early diagnosis. HIV therapy is becoming easier and more tolerable, and the benefits of increasingly earlier initiation of therapy are being studied. Research is underway to find a cure, but it is still a long way off. New drugs offer advantages in terms of tolerability and metabolic profile. However, a functioning HIV therapy should only be switched if there is a clear indication. Comedications must be evaluated for interactions with HIV medications.
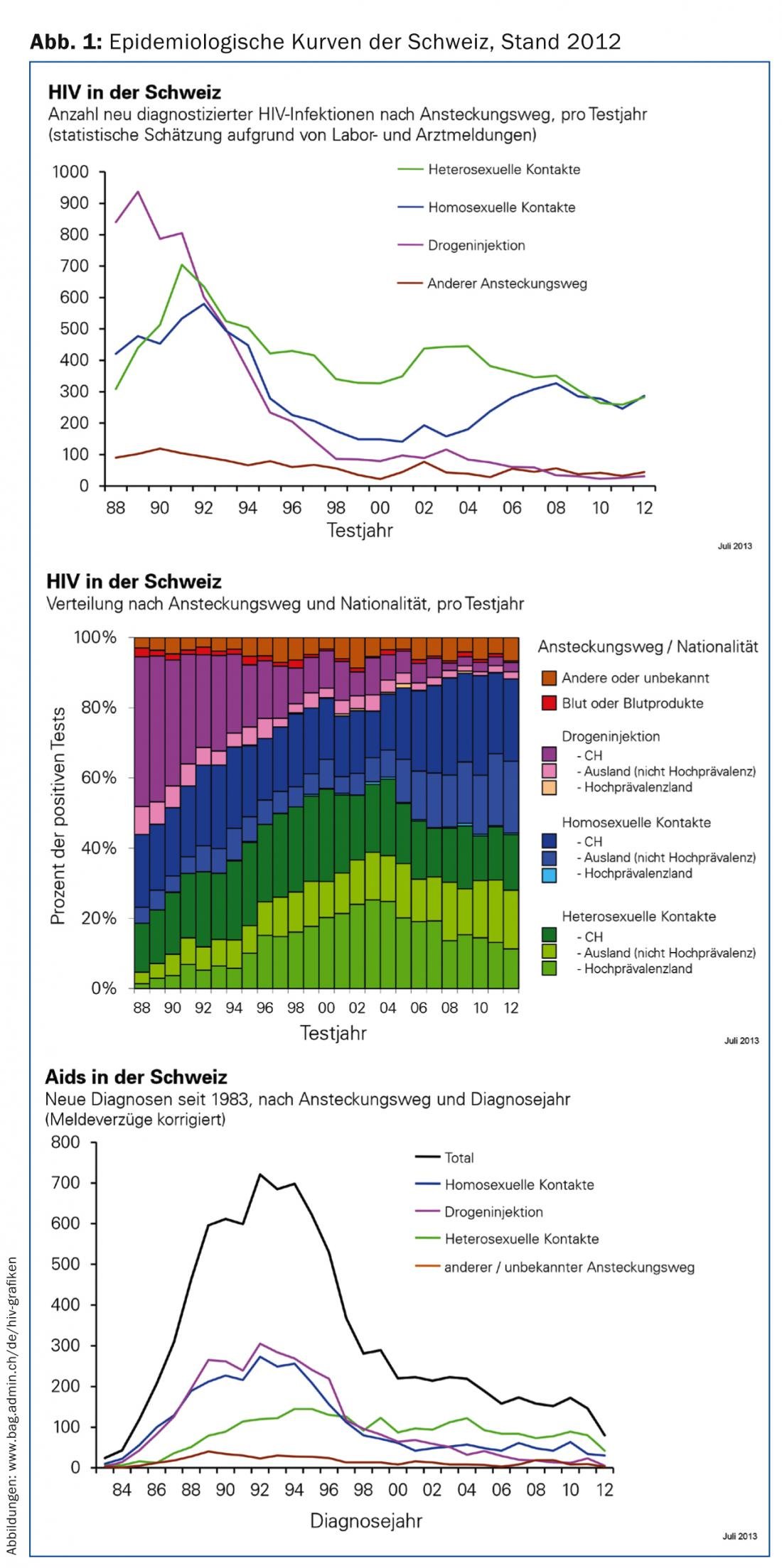
HIV incidence has not changed significantly in recent years – this in contrast to the steady increase in bacterial sexually transmitted infections since 2009. Praise should be given to the consistently very good prevention readiness of drug users, who have had the lowest incidence among the classic risk groups since the end of the 1990s (Fig. 1) [1]. With timely diagnosis, good treatment adherence, and no significant comorbidities, the life expectancy of HIV-infected patients is minimally limited [2]. Thus, based on the medical prognosis, well-controlled HIV infection is compatible with obtaining life insurance [3]. Nevertheless, each new infection is still a burden for patients and their families. Unfortunately, patients are still threatened by stigmatization and lose a piece of freedom through regular medical check-ups and daily medication from the beginning of therapy. In addition, significant costs are incurred with each new infection due to the still very high prices of drugs.
For a more comprehensive presentation of this topic, please refer to the special issue of Therapeutische Umschau from August 2014 (“HIV heute”, Volume 71, Issue 8, August 2014).
Diagnosis and basic clarifications
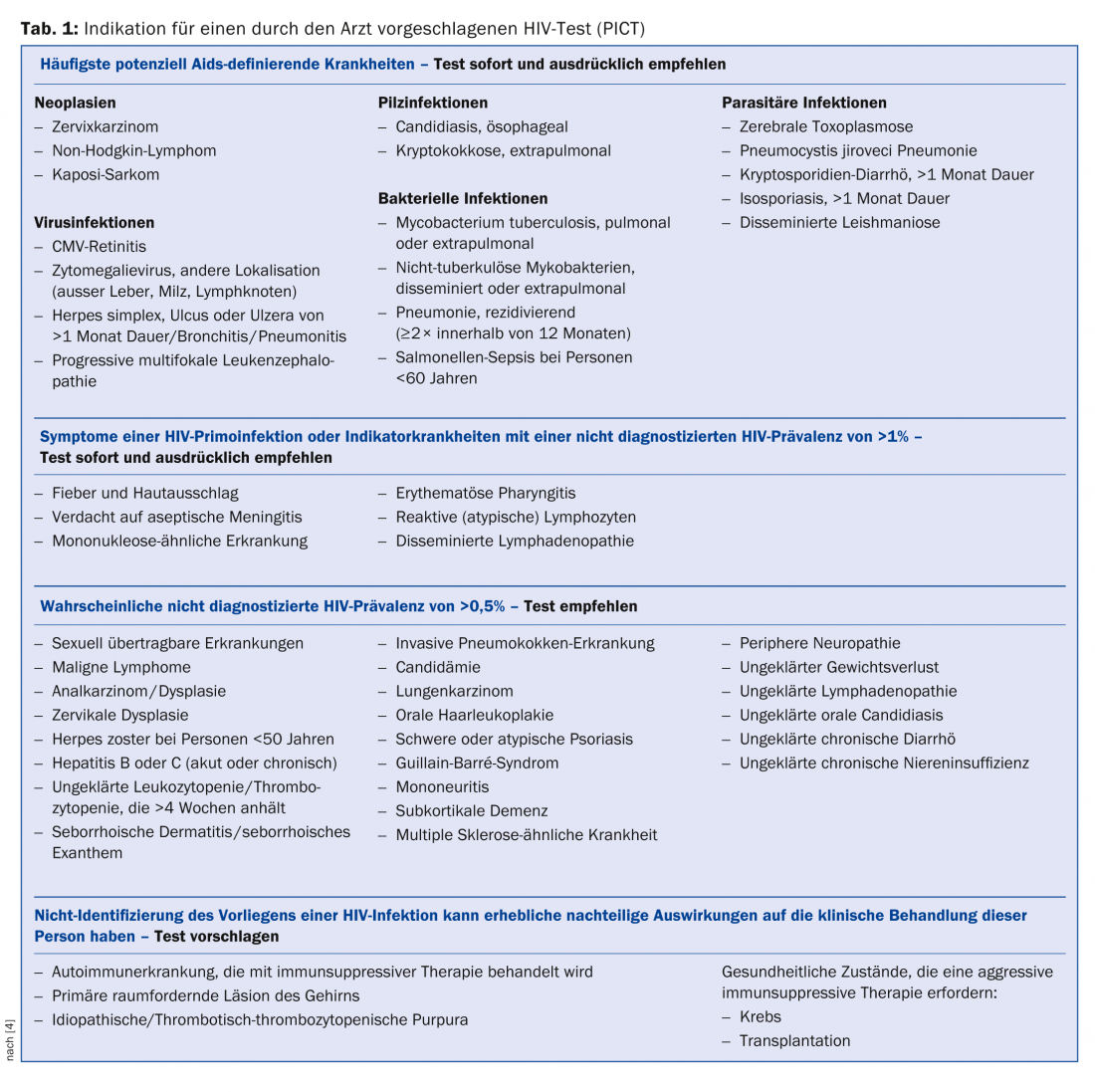
HIV testing should be offered to the following patient groups: Individuals who belong to a demographic group at increased risk for HIV (men who have sex with men [MSM], origin from sub-Saharan Africa, intravenous drug use) as well as persons who wish to undergo a check-up at their own request for sexual risk or on return from a trip (“voluntary counseling and testing”, VCT). Medical professionals should also suggest HIV testing (“provider initiated counseling and testing”, PICT) in the situations listed in Table 1 [4].
Preferred HIV testing procedure: from the first sample (capillary blood or serum/plasma), only HIV screening with combined tests (HIV-1/2 antibody and p24 antigen) is performed. If the first test is positive, a second fresh EDTA blood sample (7-10 ml) is sent directly to one of the designated HIV reporting laboratories for confirmation, along with the written result of the first test. In it are performed: Confirmation of reactivity with other assay, differentiation HIV-1/2/double infection, viral load, resistance testing and epidemiologically important differentiation between recent (<3-6 months ago) or older infections by Western blot [5].
Progress on the research front
Except for the so-called Berlin patient, who was cured of his leukemia and HIV at the same time thanks to an “HIV-resistant” bone marrow donation, there has been no sustained cure of HIV so far. Nevertheless, one basic strategy for an “HIV-cure” in particular is gaining popularity. It consists of mobilizing the dormant HIV reservoir from the body’s cells and subsequently killing the activated cells and viruses in a targeted manner. Despite promising approaches, it is still expected to take years to decades before a cure will be possible. Research has also shown that patients without therapy, even if they still have normal CD4 cells, age metabolically faster, at least as long as HIV replication and the associated inflammatory response are not suppressed. This is one reason why the indication for therapy is increasingly being made earlier.
Therapy indication
The indication for starting therapy should be discussed with a physician experienced in HIV medicine. The following are classic indications for starting antiretroviral therapy (ART):
- Emergency initiation (within one, max. two days) for symptomatic HIV primo infection or new HIV diagnosis in late pregnancy.
- As soon as possible at the onset of an AIDS-defining illness (Tab. 1): Start therapy as soon as it is stabilized.
- In asymptomatic patients, ART can be started electively (i.e., after thorough education about ART and if the patient expresses willingness to start) if patients have a CD4 cell count below 350 cells/µl. In addition, ART can be started in individual cases to prevent further infections, even with higher CD4 cells, e.g., in discordant couples or in cases of marked persistent risk behavior. This concept of “treatment as prevention” is gaining epidemiological traction. That it works was impressively documented in a randomized trial in serodiscordant couples in southern Africa [6]. However, whether early initiation of therapy in HIV-positive patients is beneficial in the long term (as already suggested in some international guidelines) will only become clear after a longer period of observation. A global study is still underway on this, the results of which will better inform this issue (insight.ccbr.umn.edu/start/).
Therapy options
First-line therapy still consists of two nucleoside reverse transcriptase inhibitors (NRTIs) and a third agent from one of the following therapeutic classes: Non-nucleoside reverse transcriptase inhibitors (NNRTI)/ritonavir-boosted protease inhibitors (r/PI)/integrase inhibitors (INI). In the last two years, new compounds and new one-pill ART combinations (“fixed dose combinations”, FDCs) have entered the market. The benefits of these new treatments include easier tablet administration, better tolerability, and a more favorable metabolic profile compared with older therapies. New substances have been approved in all substance classes in recent years:
- NNRTI: rilpivirine
- PI: Darunavir
- INI: Elvitegravir and dolutegravir
Further development of tenofovir alafenamide fumarate (TAF) is eagerly awaited. This substance is a precursor to the already known tenofovir disoproxil fumarate (TDF, Viread®), but with greater antiviral potency and accumulation in lymphoid cells, so that lower doses must be used. It is interesting to note that at the same time the concentration in the renal cells is reduced and thus significantly fewer renal and osseous side effects are to be expected than with TDF.
These new substances are very welcome additions to existing therapy. However, switching from existing virologically and immunologically successful treatments should be done only when strictly indicated. The main goal of therapy remains the sustained suppression of viral replication.
Monitoring and prevention of long-term therapy side effects.
For most patients, ART is a medication likely to be taken for decades. This requires regular monitoring with regard to possible toxicities. Lipodystrophy, mainly attributable to early ART combinations, has become much rarer. On the other hand, renal side effects (especially proximal tubulopathy) and accelerated osteoporosis development as well as dyslipidemias due to the drugs remain relevant and require regular monitoring.
Pitfall interactions
The PIs and NNRTIs have a high interaction potential, as they are metabolized via the cytochrome P450 system and themselves inhibit (PI) or induce (NNRTI) it. NRTI and INI are less problematic in this regard. In particular, levels of calcium antagonists, statins, immunosuppressants, steroids (including inhaled and injectable depot preparations), new oral anticoagulants, and hormonal anticonceptives can be significantly altered in the presence of NNRTIs and PIs. We strongly recommend consulting the interaction website (www.hiv-druginteractions.org), the Drug Compendium (www.compendium.ch), or an HIV specialist before any change in therapy in patients on ART.
PEP – the most important old and new points
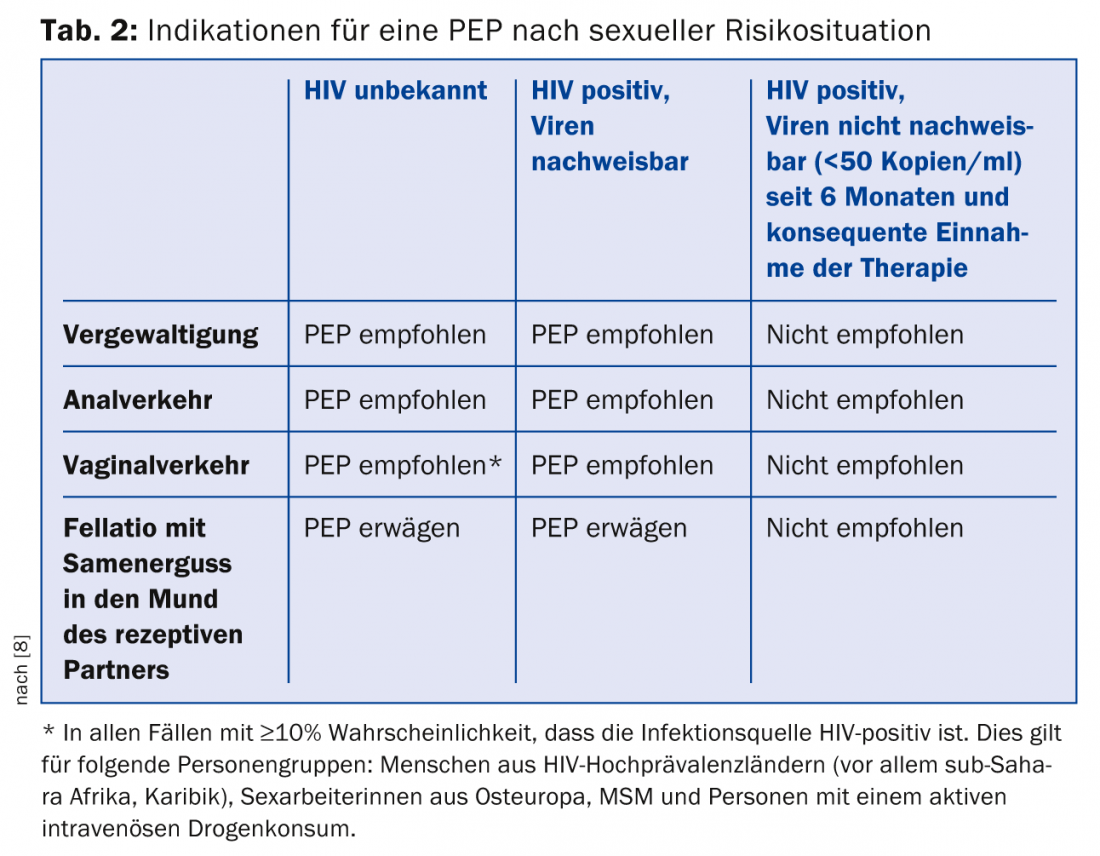
The decision to use postexposure prophylaxis (PEP) with a triple antiretroviral combination after confirmed or possible contact with HIV is an emergency that requires an accurate risk assessment. Basically, the two main situations in which PEP may occur are handled differently in terms of risk assessment: on the one hand, exposure to potentially infectious blood in persons in health care professions with guidelines dating from 2007 [7]. Second, PEP should also be considered after relevant sexual risk contacts; the relevant guidelines were revised last year [8].
Important changes: For sexual risk contacts, only a 48-hour window now applies for the start of PEP. For the time being, a 72-hour window continues to apply for occupationally indicated PEP. PEP should be newly administered with one of the following ART combinations during four weeks:
- Truvada® (tenofovir/emtricitabine) 1×/d plus Isentress® (raltegravir) 2× 400 mg/d.
- Truvada® (tenofovir/emtricitabine) 1×/d plus Tivicay® (dolutegravir) 50 mg 1×/d
- Truvada® (tenofovir/emtricitabine) 1×/d plus Prezista® (darunavir) 800 mg.
1×/d plus Norvir® (ritonavir) 100 mg 1×/d
In individual cases, the PEP may be modified after obtaining expert opinion. For information on who should be given a PEP, see Table 2.
PrEP – Pre-exposure prophylaxis before sexual risk situations
Truvada® (tenofovir/emtricitabine) has shown efficacy in PrEP in several studies. On 10/29/2014, the French IPERGAY trial [9] of Truvada® was stopped early due to convincing efficacy. A requirement for functioning is taking at least four tablets in a row for infrequent episodic exposure and at least four tablets per week for virtually continuous use. Because of its high cost, PrEP is not yet covered by health insurance and remains the preserve of a minority with appropriate risk behavior and financial resources. Injectable depot preparations could offer an alternative in the future.
Literature:
- Federal Office of Public Health: HIV and AIDS in Switzerland: Graphics. 2013. www.bag.admin.ch/de/hiv-grafiken (available in all four national languages).
- Sabin CA: Do people with HIV infection have a normal life expectancy in the era of combination antiretroviral therapy? BMC Med 2013; 11: 251.
- Kaulich-Bartz J, et al: Insurability of HIV-positive people treated with antiretroviral therapy in Europe: collaborative analysis of HIV cohort studies. AIDS 2013; 27(10): 1641-1655.
- Federal Office of Public Health: The HIV test on the initiative of the physician for certain clinical pictures (HIV indicator diseases). FOPH Bulletin 18 November 2013. www.bag.admin.ch/hiv_aids/05464/12752/index.html?lang=de
- Federal Office of Public Health: The Swiss testing concept – an updated overview. 2013. www.bag.admin.ch/hiv_aids/05464/12752™/index.html?lang=en
- Cohen MS, et al: Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med 2011; 365(6): 493-505.
- Federal Office of Public Health: Procedure after exposure to blood or other biological fluids of health care workers – updated recommendations. 2007. www.bag.admin.ch/hiv_aids/05464/12752/index.html?lang=de
- Federal Office of Public Health: Emergency HIV exposure – PEP can be the right response. 2014. www.bag.admin.ch/hiv_aids/05464/12752/index.html?lang=de
- Molina JM, ANRS. Ipergay Study. 2014. %20release%
HAUSARZT PRAXIS 2015; 10(2): 13-16