Renal cell carcinoma is one of the common malignant tumors in adults. In terms of drug treatment options, this tumor entity has undergone more rapid change in recent years than almost any other malignant disease. Meanwhile, the focus is not only on targeted therapy in advanced renal cell carcinoma, but also on combination treatments.
Kidney cancer is the third most common urologic cancer. Every year, nearly 1000 people in Switzerland develop a malignant renal tumor [1]. The majority of tumors histologically belong to the clear cell renal carcinomas, which – depending on the subtype – have a slightly better prognosis than non-clear cell renal cancer [2]. However, in the advanced stage, the prognosis is unfavorable for most affected individuals: three quarters of them have a moderate to high risk of the disease not stopping [3]. Numerous new approvals have significantly changed the therapeutic landscape for metastatic renal cell carcinoma. Targeted therapies were joined by immune checkpoint inhibitors, which can now be used in combination [4].
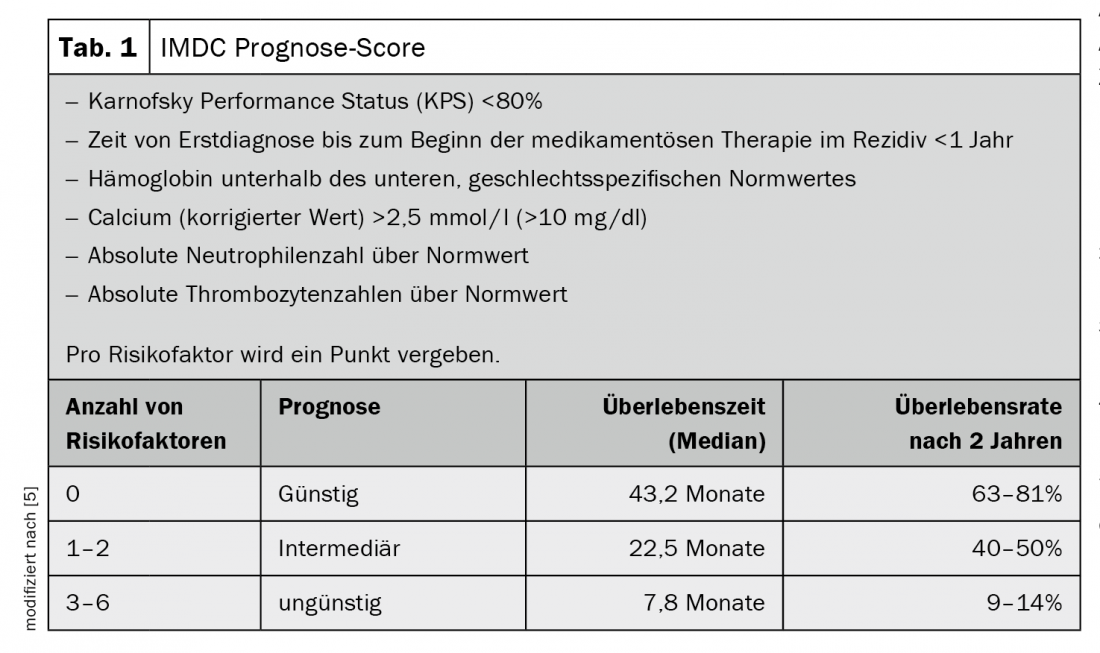
Targeted therapy with tyrosine kinase inhibitors, mTOR inhibitors or antibodies has become established in recent years. Most notably, vascular endothelial growth factor receptor (VEGFR) inhibition is the standard option for patients with favorable prognosis whose tumors have failed surgery or have metastasized [5] (Tab. 1). The first checkpoint inhibitor, targeting the surface protein PD-1, was then approved as second-line therapy after failure of prior therapy. Recently, dual immune checkpoint blockade has also become possible in earlier use. The combination therapy of the checkpoint inhibitor nivolumab with the antibody ipilimumab directed against CTLA-4 was approved. Compared with targeted therapy, dual checkpoint blockade shows advantages in patients with intermediate or unfavorable baseline risk. At 18 months, 75% of those affected were still alive and median overall survival was not achieved during the study observation period. In 9% of those treated with immunotherapy, the disease responded completely to treatment, and the tumor was therefore initially undetectable after therapy [3]. However, the chance of achieving an optimal response and a long-lasting remission with the new therapeutics is accompanied by a high rate of side effects. Their treatment requires a well-coordinated interdisciplinary treatment team [4].
The future: combination therapies
A new way of immuno-oncological treatment of renal cancer is to combine the established targeted substances with the still relatively new checkpoint inhibitors. In America, the new mode of action consisting of, for example, the tyrosine kinase inhibitor axitinib with pembrolizumab (anti-PD-1 antibody) or axitinib with the also anti-PD-L1 antibody avelumab has already been approved. In Europe, approval is eagerly awaited.
Literature:
- www.krebsliga.ch/ueber-krebs/zahlen-fakten/-dl-/fileadmin/downloads/sheets/zahlen-krebs-in-der-schweiz.pdf (last accessed 15.30.2020)
- www.krebsinformationsdienst.de/fachkreise/nachrichten/2019/fk17-nierenkrebs-immuntherapie-kombination.php (last accessed 15.30.2020)
- Motzer RJ, Nizar M, Tannier MD, et al: Nivolumab plus ipilimumab versus sunitinib in advanced renal cell carcinoma. N Engl J Med 2018; 378: 1277-1290.
- Zschäbitz S, Ivanyi P, Delecluse S: Revolution in systems therapy of metastatic renal cell carcinoma. The Nephrologist 2020; 15: 12-19.
- Ivanyi P, Grünwald V: Systems therapy of renal cell carcinoma. Oncologist 2019; 25: 517-522.
- Heng DYC, Xie W, Regan MM, et al: External validation and comparison with other models of the International Metastatic Renal-Cell Carcinoma Database Consortium prognostic model: a population-based study. Lancet Oncol 2013; 14: 141-148.
InFo ONCOLOGY & HEMATOLOGY 2020; 8(2): 23.