More recently, studies have shown that aspirin improves overall survival in subgroups with colorectal cancer, specifically those with PIK3CA mutations. To test whether regular aspirin use is associated with survival in metastatic colorectal cancer, a study presented at ASCO GI in San Francisco collected data from two large academic institutions. In addition, experts discussed the impact of KRAS-NRAS status on second-line panitumumab therapy in patients with metastatic colorectal cancer.
(ag) The study, which examined the effects of regular aspirin use on metastatic colorectal cancer (CRC) or survival, was presented by Nishi Kothari, Tampa, at ASCO GI in San Francisco: “From two large academic institutions, namely the Moffitt Cancer Center in Tampa and the Royal Melbourne Hospital in Australia, we identified patients with PIK3CA-mutated CRC.”
In the two research centers, PIK3CA mutations were detected by exon sequencing (entirety of all exons). Prospective data, including age, sex, and disease location, as well as survival data, were available to the research team.
What can aspirin actually do?
In 2010, the best-known proponent of the “aspirin theory,” Peter M. Rothwell, compiled data from more than 14,000 CRC patients and concluded that aspirin not only reduced the incidence of this cancer by 24%, but also reduced its mortality by 35% [1]. In late 2012, Liao et al. concluded in their study [2] that regular aspirin use after CRC diagnosis was associated with longer survival, and only in patients with PIK3CA mutations. The values here reached statistical significance for both cancer-specific (p<0.001) and overall survival (p=0.01). Can these results be confirmed for PIK3CA-mutated metastatic CRC?
Kothari’s study identified a total of 187 CRC patients with a median age of 72 years and confirmed PIK3CA mutations, 26% of whom were taking aspirin regularly. One quarter of the participants suffered from metastatic disease at the time of diagnosis. Median follow-up was 48 months.
In univariate analyses of data from all subjects, aspirin use was not associated with significantly better overall survival (p=0.6), but there was a trend toward better cancer-specific survival (p=0.06).
No improvements at all in either overall or cancer-specific or recurrence-free survival were seen in patients with stage II or III disease (AICC classification).
However: in those at stage IV, regular aspirin use was indeed significantly associated with prolonged overall survival (p=0.04). A statistically significant association was also found for cancer-specific survival (p=0.02).
Significant improvement only in advanced disease stages
“What conclusion can be drawn from this recent study on aspirin in cancer therapy?” wondered Kothari in conclusion. “Well, our study is probably the largest investigation to date looking at regular aspirin use in PIK3CA-mutated metastatic CRC. It clearly shows that regular aspirin use may be associated with prolonged survival. However, not in all disease stages, but only in the more advanced ones.”
Of course, crucial limitations of the study should not go unmentioned: The results were not confirmed in the multivariate analysis. In addition, the analysis was retrospective with respect to aspirin use and information on therapy. Follow-up was also limited. “Our study should ultimately provide impetus to investigate the issue more deeply in prospective designs,” Kothari concluded.
RAS mutation status and panitumumab.
According to Marc Peeters, Edegem, large-scale studies have recently demonstrated two aspects of taking panitumumab (anti-EGFR) in the treatment of metastatic CRC: In combination with FOLFIRI (fluorouracil, leucovorin, and irinotecan), it highly significantly improves progression-free survival compared with FOLFIRI alone (p=0.004) and shows a trend toward better overall survival (p=0.12) [3]. Second, another analysis [4] found that mutations in several RAS genes (KRAS/NRAS, exon 2, 3, 4) may predict nonresponse to panitumumab plus FOLFOX4 (oxaliplatin, fluorouracil, and leucovorin). Consequently, the inclusion of other mutations, complementary to the KRAS exon 2 status, seems crucial to predict therapeutic success.
“Our goal now was to investigate the extent to which treatment success in metastatic CRC is related to RAS status. Specifically, we were looking at progression-free and overall survival with panitumumab-FOLFIRI in the second-line setting,” Peeters said. “Patients with mutant RAS were found to benefit less from panitumumab addition (Table 1).
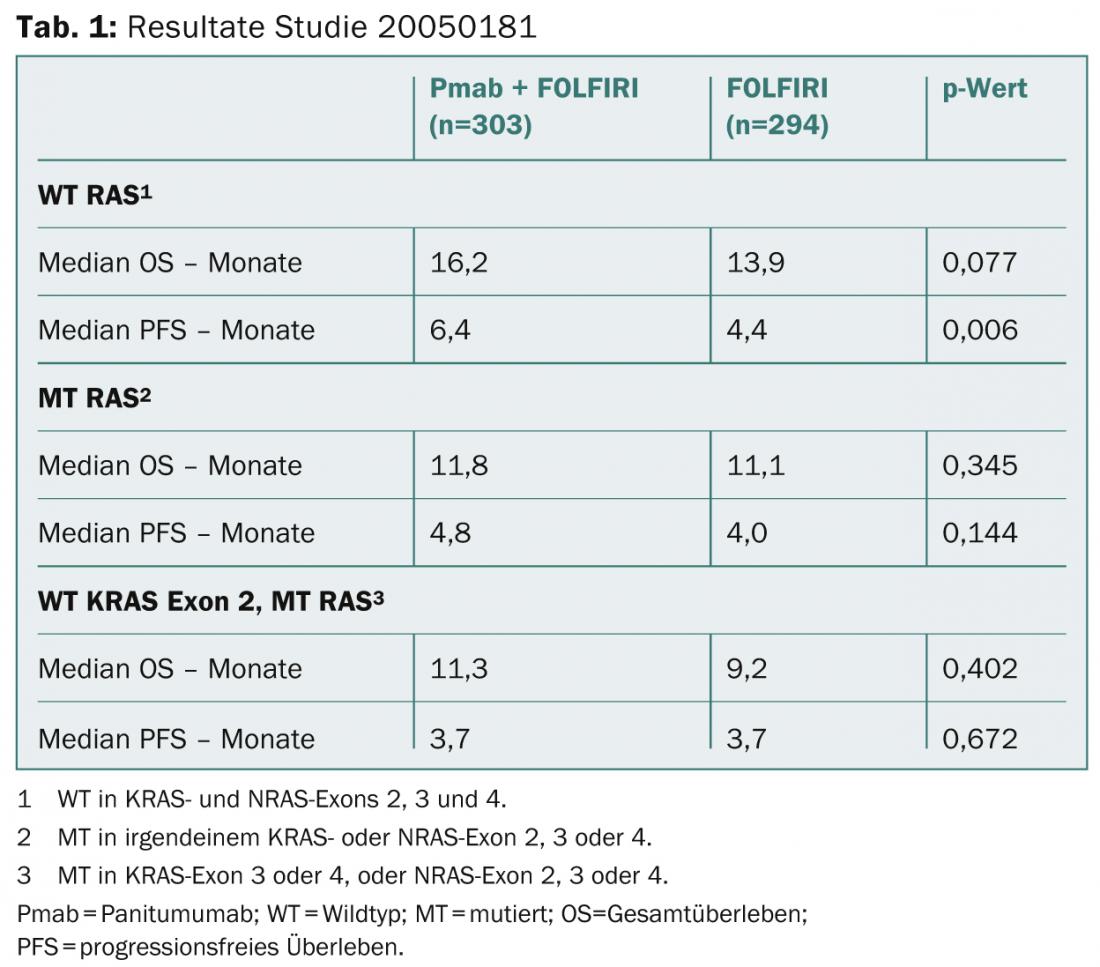
Not surprisingly, the same is true for those with mutated KRAS exon 2, which is, after all, already an established predictive biomarker. Of great relevance is the finding that participants with mutations in other RAS genes and a Kras exon 2 wild type also responded poorly to panitumumab. In addition, therapeutic success further improved when not only KRAS exon 2 but all RAS genes were wild type. Accordingly, our results are consistent with previous studies. The selection of patients for panitumumab therapy should be made by RAS testing,” Peeters concluded.
Source: “Cancers of the Colon and Rectum,” Oral Abstract Session at ASCO GI – Gastrointestinal Cancers Symposium, January 16-18, 2014, San Francisco.
Literature:
- Rothwell PM, et al: Long-term effect of aspirin on colorectal cancer incidence and mortality: 20-year follow-up of five randomised trials. Lancet 2010 Nov 20; 376(9754): 1741-1750. doi: 10.1016/S0140-6736(10)61543-7. epub 2010 Oct 21.
- Liao X, et al: Aspirin use, tumor PIK3CA mutation, and colorectal-cancer survival. N Engl J Med 2012 Oct 25; 367(17): 1596-1606. doi: 10.1056/NEJMoa1207756.
- Peeters M, et al: Randomized phase III study of panitumumab with fluorouracil, leucovorin, and irinotecan (FOLFIRI) compared with FOLFIRI alone as second-line treatment in patients with metastatic colorectal cancer. J Clin Oncol 2010 Nov 1; 28(31): 4706-4713.
- Douillard JY, et al: Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. N Engl J Med 2013 Sep 12; 369(11): 1023-1034. doi: 10.1056/NEJMoa1305275.
InFo Oncology & Hematology 2014; 2(2): 22-23.











