Topical therapies are still standard treatment for mild psoriasis and should be used concomitantly with phototherapy or systemic therapy for moderate to severe forms. At the 2019 EADV Congress, renowned experts provided an up-to-date overview.
Basic care and topical therapies now play an important role in the management of psoriasis, emphasized Prof. Wolf-Henning Boehncke, MD, University of Geneva [1,2]. While this is the mainstay of therapy for mild symptoms, combined use with systemic treatment has proven effective for severe forms of the disease.
According to European consensus, mild psoriasis is defined as follows: BSA (Body Surface Area) ≤10%, PASI (Psoriasis Area and Severity Index) ≤10 points, DLQI (Dermatological Quality of Life Index) ≤10 points. Topical treatment has a high value in the daily routine of dermatologists, as the majority of patients suffer from psoriasis of mild severity [3]. Systemic treatment of mild psoriasis may be considered when topical therapy does not result in sufficient symptom reduction or when upgrade criteria are present, such as infestation of visible areas, the genital region, in the presence of particular symptoms such as very severe itching or nail infestation [3].
Define therapy goals
The establishment of success criteria serves as the basis for monitoring the progress and evaluation of the treatment. Complete or near freedom from appearance is a goal to strive for, but not always realistic. It is suggested to perform a follow-up of the topical therapy initially after four to eight weeks and every eight to twelve weeks during the maintenance therapy [3]. In addition to symptom classification by PASI and BSA scores, performing a physician global assessment of severity (PGA, Physican Global Assessment) is also recommended. Patient satisfaction can be assessed with the DLQI (Dermatological Life Quality Index) questionnaire. Shared decision making with the patient about planned therapy and expected treatment outcome has been shown to increase adherence, thereby increasing the likelihood of a positive response. A personalized approach also includes the inclusion of empirical values and personal preferences of the patient. Moreover, a therapy regimen that is as simple as possible (e.g., once-daily application) has been shown to promote compliance.
Standard therapy in the initial phase is still considered to be once-daily treatment with a fixed combination of calcipotriol 50 μg/g and betamethasone dipropionate 0.5 mg/g for 4-8 weeks [4]. The choice of delivery method (lipid gel vs. foam) should also be based on patient preferences. After the induction phase, application once or twice a week is usually sufficient. Alternatively, class II and class III steroids and vitamin D analogues can be used as monotherapy or intermittently.
Proven product selection
In addition to relieving pruritus and scaling skin, topical topical preparations can lead to a reduction in exacerbations and a variety of other positive effects, which is an important contribution, particularly in the maintenance phase, and supports phases of remission (Tab. 1) . This can have a positive impact on the quality of life of those affected. Glucocorticoids, vitamin D derivatives and calcineurin inhibitors are the pillars of topical therapy and there are also some new active substances in the pipeline. In some cases, a combination of topical therapy and narrow-spectrum UVB may result in symptom reduction.
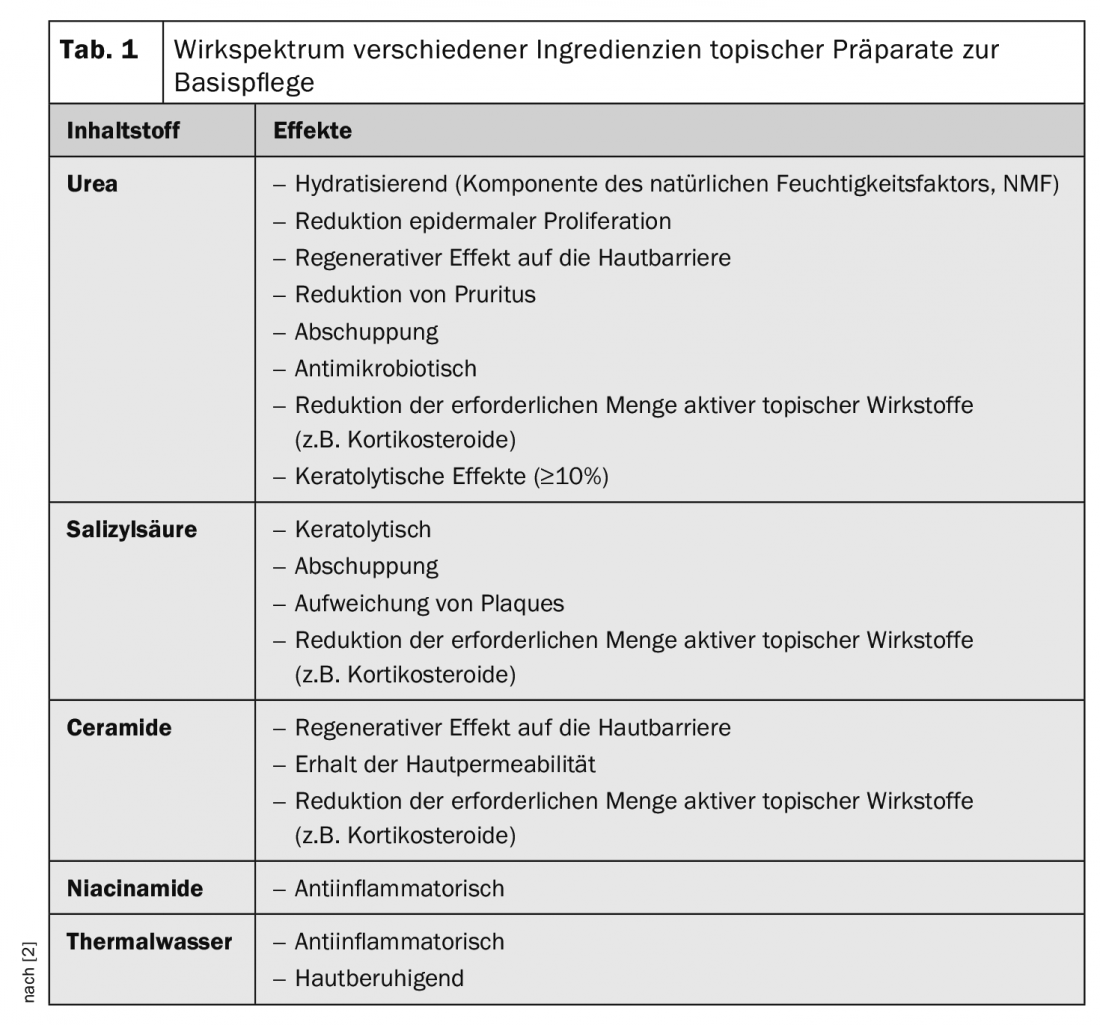
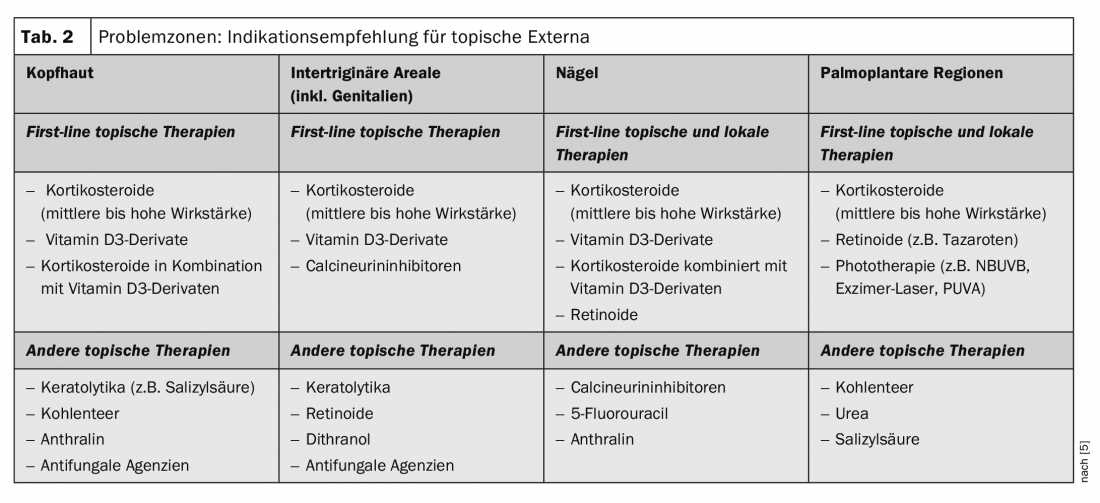
Despite major advances in therapeutic options for psoriasis, difficult-to-treat areas including scalp, nails, intertriginous areas (including genitalia) and palmoplantar regions remain a major challenge. This is associated with corresponding stresses for patients. In a review published in 2018, a classification of the most appropriate topical topical externals was made based on current data for these problem areas (Table 2) [2,5].
Future treatment options?
Tofacitinib is a small molecule Janus kinase inhibitor being investigated for the topical treatment of psoriasis. In a phase IIb study, this agent showed superior efficacy compared with placebo in adults with mild to moderate plaque psoriasis – applied in a 2% formulation (ointment) 1× or 2× daily – at 8 weeks, but at week 12 after baseline, the difference was no longer significant [6]. The safety profile was satisfactory. The primary end point was the proportion of patients with a PGA-C (Calculated Physicians Global Assessment) who were lesion-free or nearly lesion-free at 8 and 12 weeks after baseline and achieved ≥2 improvement in symptom score. The sample included 430 subjects.
Currently, various strategies of innovative galenics are being evaluated, with the help of which, among other things, small-molecule systemic anti- psoriatic drugs (e.g. methotrexate) are to be rapidly and specifically transported into psoriasis plaques after topical application, e.g. by coupling to biologics and/or “packaging” in nanoparticles [7].

Literature:
- Thaçi D, et al: Importance of basic therapy in psoriasis. JDDG 2015; 13(5): 415-418.
- Boehnke W-H: Topical therapy and phototherapy. Slide presentation, Prof. Wolf-Henning Boehnke, MD, Psoriasis Training and Education Forum, EADV Congress, Madrid, Oct. 11, 2019.
- Körber A, et al: Topical therapy for psoriasis vulgaris – a treatment pathway. JDDG 2019; 17 (S4): 3-14. https://onlinelibrary.wiley.com/doi full/10.1111/ddg.13810
- von Kiedrowski R, et al: Recommendations for the ambu lant care of psoriasis vulgaris. Updated practice-based treatment pathway. www.onkoderm.de
- Kivelevitch D : Pharmacotherapeutic approaches for treating psoriasis in difficult-to-treat areas. Expert Opinion on Pharmacotherapy 2018; 19(6): 561-575.
- Papp KA, et al: Treatment of plaque psoriasis with an ointment formulation of the Janus kinase inhibitor, tofacitinib: a phase 2b randomized clinical trial. BMC Dermatol. 2016; 16(1): 15.
- Ferreira M, et al: Topical co-delivery of methotrexate and etanercept using lipid nanoparticles: A targeted approach for psoriasis management. Colloids Surf B Biointerfaces 2017; 159: 23-29.
DERMATOLOGIE PRAXIS 2019; 29(6): 35-36 (published 12/6/19, ahead of print).