Since the beginning of June this year, tralokinumab – a human monoclonal IgG4 antibody that specifically binds with high affinity to the cytokine IL-13 – has been approved for adults with atopic dermatitis in Switzerland. Both the efficacy data and the safety profile of this biologic are extremely promising according to clinical studies. In patients who show a rapid response, a four-week dose interval may be considered.
Atopic dermatitis is one of the type 2 dominant diseases caused by complex interactions between genetic factors and environmental influences. The understanding of etiopathogenesis has expanded fundamentally in recent years. This has led, among other things, to the development of modern system therapies from the substance classes of biologics and Janus kinase inhibitors, which is of great significance because moderate and severe forms of atopic dermatitis in particular are associated with a high disease burden. Severe itching and visible eczematous skin lesions are often associated with depressed mood, anxiety, and impaired sleep quality. An important treatment goal is to improve quality of life, which is most likely to be achieved by treatment options that have both a high efficacy and a favorable safety profile. “We in the practice expect treatments that are safe and easy to use,” explains PD Ahmad Jalili, MD, Dermatology & Skin Care Clinic, Buochs [1]. The fact that the use of biologics does not require prescreening and usually only a few laboratory tests is a great advantage in view of the time pressure in everyday clinical practice.
Th2-mediated inflammation is pathophysiologically central
Atopic dermatitis usually begins in early childhood, but sometimes in adulthood. Itching and resulting scratching contributes to skin-related complications such as excoriation and infection [2,3]. The pathogenesis of atopic dermatitis is complex and involves multiple pathways. “Today, you can translate this into therapies and that’s why this has become increasingly important,” said Prof. Peter Schmid- Grendelmeier, MD, of the Department of Dermatology, University Hospital Zurich [3]. Thus, there has been great progress in recent years in the study of pathogenesis and in the development of treatment strategies based on it. The major pathogenetic factors in atopic dermatitis include Th2-mediated inflammation and an epidermal barrier defect. Cascades take place that maintain and intensify itching and irritate the nerve fibers of the skin, explains the expert. From studies of immunopathological mechanisms in atopic dermatitis, it is clear that cytokines IL-4 and IL-13 produced by Th2 cells in particular are of central pathophysiological importance [4]. One therapeutic strategy to inhibit the proinflammatory activity of these messengers is monoclonal antibodies that inhibit signaling [5,6].
Tralokinumab selectively inhibits the key cytokine IL-13
As the first representative of biologics in the indication area of atopic dermatitis, the monoclonal antibody Dupilumab, which is directed against IL-4 and IL-13, was launched in Switzerland in 2019. With the recently approved tralokinumab, another biologic is now available. “We are very happy to now have a second substance,” said Prof. Schmid-Grendelmeier. Adtralza® (tralokinumab) may be used in adult patients with moderate to severe atopic dermatitis when therapy with prescription topical medications does not provide adequate disease control or is not recommended [7]. Tralokinumab specifically neutralizes IL-13 by binding to this cytokine, preventing its interaction with the receptor IL-13Rα1 [6,8]. IL-13 is a key cytokine in the pathogenesis of atopic dermatitis [6]. Overexpression of IL-13 has been found in both lesional and non-lesional atopic skin and, moreover, IL-13 levels have been found to correlate with the severity of atopic dermatitis [9].
Improvement of the skin condition and relief of itching
“The most important studies for approval were ECZTRA 1-3,” explains Prof. Dr.med. Stephan Weidinger of the Center for Inflammatory Skin Diseases, University Hospital Schleswig-Holstein, Kiel Campus [10]. ECZTRA 1 (n=802) and ECZTRA 2 (n=794) evaluated the efficacy and safety of monotherapy with tralokinumab in moderate to severe atopic dermatitis in two identically designed 52-week multinational randomized-controlled phase III trials [11]. Adult study participants were randomized in a 3:1 ratio to tralokinumab 300 mg every 2 weeks (q2w) or placebo. In both studies, tralokinumab was significantly superior to placebo in terms of improvements in the primary endpoints IGA 0/1 and EASI-75 at 16 weeks [11–13]. “We need treatments that not only improve the skin but also relieve itching, and tralokinumab does that,” said Prof. Weidinger [10]. In addition to weekly scores for pruritus, significant improvements were also seen in other secondary endpoints such as DLQI and eczema-related sleep disturbance [8,11]. In patients who respond rapidly to tralokinumab treatment, extension of the injection interval to four weeks (q4w) may be considered (Box). It was also shown that many of the patients who had not met the IGA 0/1 or EASI-75 endpoints at week 16 with tralokinumab q2w improved when treatment was continued [11]. “Many of the patients who initially have only a partial response to therapy, i.e. show only some improvement in the first 16 weeks, then develop the desired disease control with continued therapy,” summarizes Prof. Weidinger.

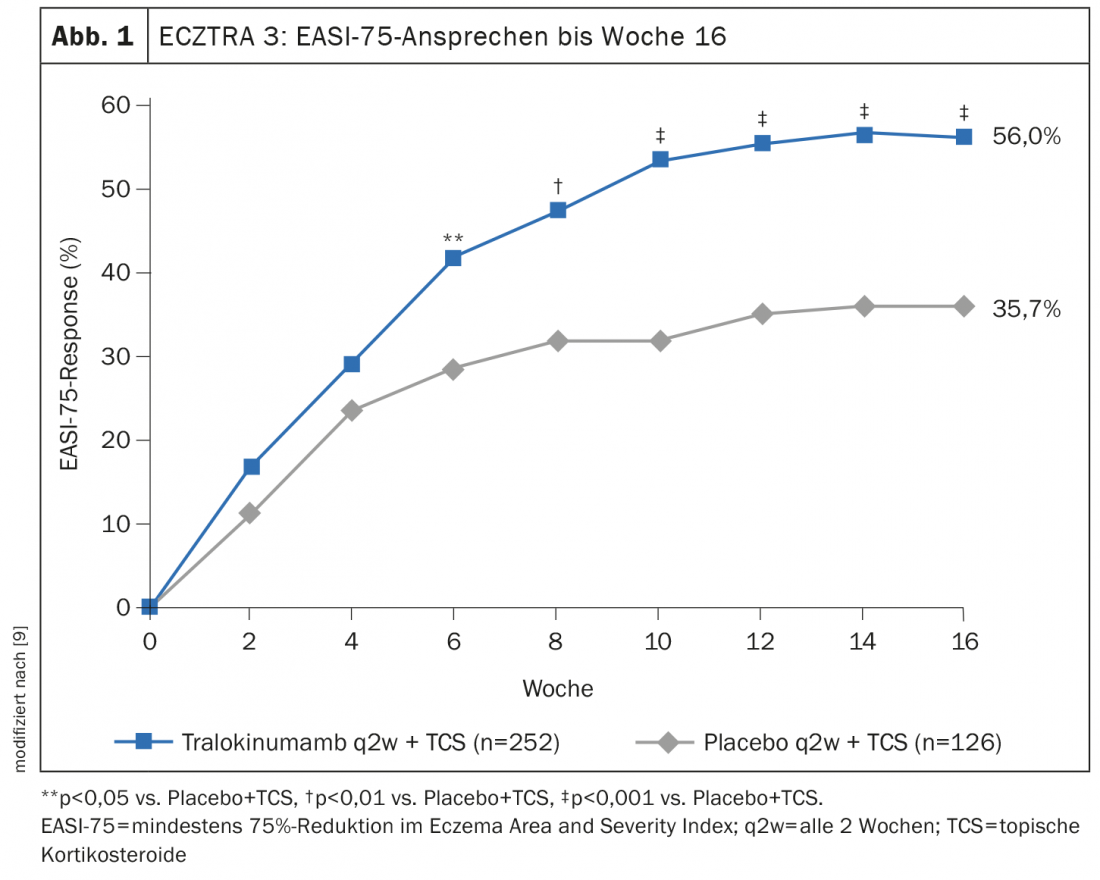
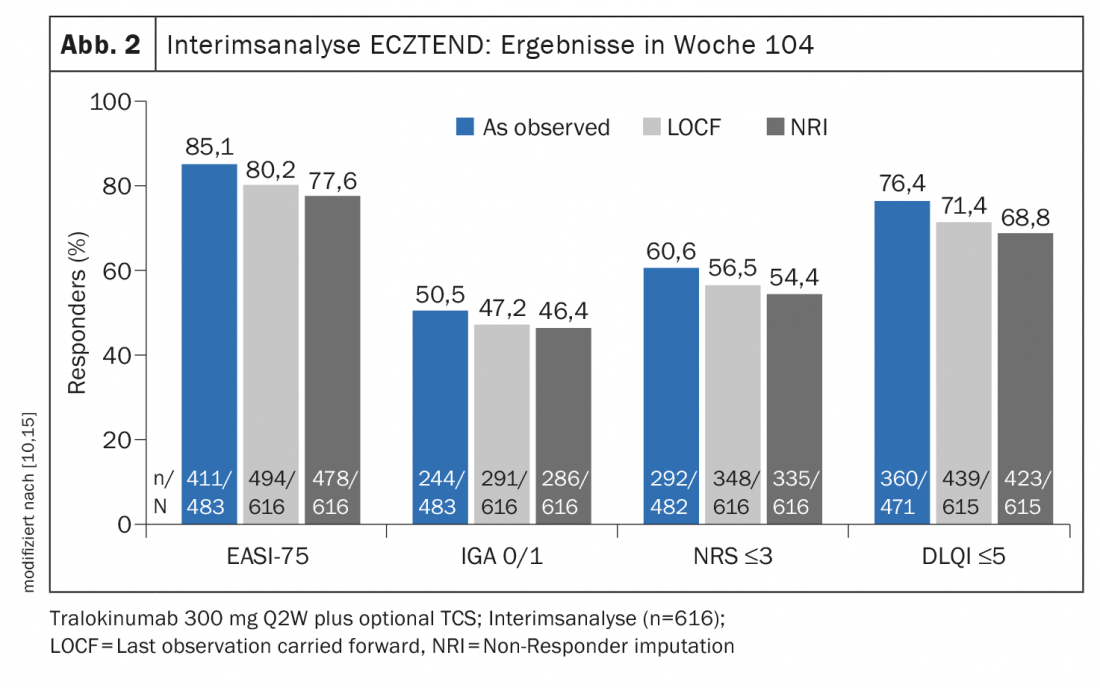
ECZTRA 3 evaluated the efficacy and safety of tralokinumab 300 mg q2w in combination with a topical corticosteroid* (TCS) in 380 patients with moderate to severe atopic dermatitis [9]. At week 16, significantly more achieved EASI-75 in the tralokinumab arm (56.0%) compared to placebo (35.7% ) (Fig. 1). And in terms of IGA 0/1, tralokinumab also proved significantly superior to placebo (38.9% vs. 26.2%). Those who met the primary endpoint at week 16 were again randomized 1:1 to continue tralokinumab q2w treatment or to reduce dosing to q4w. Approximately 90% of patients maintained response through week 32 with both dosing regimens [8]. The long-term treatment effects of tralokinumab are being investigated in the open-label extension study ECZTEND in over 1400 patients with atopic dermatitis. In interim analyses, of 616 patients treated for 2 years (104 weeks) with tralokinumab q2w plus optional TCS achieved sustained improvements in the extent and severity of atopic dermatitis (Fig. 2) [10,15].
* The on-demand therapy with locally applied cortisone was mometasone furoate 0.1% cream (class III steroid), applicable once daily to active lesions.
Long-term data underscore the excellent benefit-risk profile
The only notable adverse side effect of tralokinumab in clinical trials was the occasional occurrence of conjunctivitis, which is also a known side effect of dupilumab. In ECZTRA- 1 and -2, 7% and 3% of tralokinumab-treated patients, respectively, were affected. Most often, it is mild or moderate conjunctivitis, the speaker said. Initial ECZTEND safety data confirm that tralokinumab is generally very well tolerated [14]. In summary, these results show that the benefit-risk profile of tralokinumab is very good, even over longer treatment periods, summarizes Prof. Weidinger.
Literature:
- “High disease burden- what does atopic dermatitis mean for the patient?”, PD. Ahmad Jalili, MD, PhD, Adtralza Press Roundtable, Leo Pharma, 06/01/2022.
- Narla S, Silverberg JI: Ann Allergy Asthma Immunol 2018; 120(1): 66-72.e11.
- “Therapeutic landscape of AD in Switzerland – the future with Adtralza”, Prof. Dr. med. Peter Schmid-Grendelmeier, Press Roundtable Adtralza, Leo Pharma, 01.06.2022.
- Bieber T, et al: JDDG 2019 17; 11: 1150-1163.
- Bieber T: Allergy 2020; 75: 54-62.
- Schmid-Grendelmeier P: Dermatology Practice 2021; 31(4): 10-14.
- Swissmedic: Medicinal product information, www.swissmedicinfo.ch, last accessed 10.08.2022
- Wollenberg A, et al: J Dtsch Dermatol Ges 2021; 19(10): 1435-1442.
- Silverberg JI, et al: Br J Dermatol 2021; 184: 450-63.
- “From data to clinic: my tralokinumab experience,” Adtralza Press Roundtable, Prof. Stephan Weidinger, MD, PhD, Adtralza Press Roundtable, Leo Pharma, June 01, 2022.
- Wollenberg A, et al: Br J Dermatol 2021; 184: 437-449.
- https://clinicaltrials.gov/ct2/show/NCT03131648
- https://clinicaltrials.gov/ct2/show/NCT03160885
- Wollenberg A, et al: DDG Kompakt 2022, abstract volume, P030.
- Blauvelt A, et al: Poster presentation at AAD 2022, 25-29 March 2022.
DERMATOLOGIE PRAXIS 2022; 32(4): 42-43