Histology (tumor resection or biopsy) is essential for diagnosis and evaluation of prognosis and therapy in higher grade gliomas. Molecular markers serve as an adjunct to WHO classification regarding expected disease progression and treatment response to alkylating chemotherapy. Standard therapy for patients younger than 65 or 70 years consists of combined radiotherapy and chemotherapy with concomitant and adjuvant temozolomide (TMZ). Patients over 65 or 70 years of age have a survival advantage with chemotherapy with TMZ in the case of a methylated MGMT promoter; without a methylated MGMT promoter, radiotherapy alone is recommended. In anaplastic oligodendrogliomas and oligoastrocytomas with 1p/19q codeletion, there is a survival benefit in the case of early alkylating chemotherapy (PCV or TMZ).
Anaplastic gliomas and glioblastomas are among the most common malignant brain tumors. They are characterized by an unfavorable prognosis. Histological confirmation via surgical tissue biopsy is necessary to establish the diagnosis. For all malignant gliomas, a high extent of resection should be aimed for, as far as possible, because of the more favorable prognosis, without accepting a permanent neurological deficit. Brain tumors are classified into grade I to IV according to the WHO classification (2007) based on anatomical-pathological criteria. The aim of this classification is to provide guidance on prognosis, clinical course, and evidence-based treatment options based on the degree of malignancy.
Molecular markers
In recent years, molecular markers have been identified that characterize subtypes of gliomas with different prognosis and response to therapies. A marker with prognostic value informs about the prognosis of the disease independent of the therapy performed. Particularly relevant to clinical decision-making is a marker with predictive value that predicts response to a particular therapy.
- 1p/19q codeletion: A molecular marker closely associated with oligodendroglial tumors is the combined loss of chromosome arms 1p and 19q (“loss of heterozygosity”, LOH-1p/19q, 1p/19q codeletion).
- MGMT promoter: Another important marker is the methylation status of the O6-methylguanine-DNA methyltransferase (MGMT) promoter. MGMT is a DNA repair protein that reduces the effects of alkylating chemotherapy. In the case of promoter methylation, the MGMT gene is diminished in expression, thus relatively improving the efficacy of alkylating chemotherapy. A methylated MGMT promoter has prognostically favorable value for WHO grades III and IV gliomas and, depending on the subgroup, is also predictive in terms of a survival advantage in the case of alkylating chemotherapy.
- IDH mutation: a prognostically favorable marker across all gliomentities is a mutation in the isocitrate dehygenase(IDH)-1 or -2 gene. Patients with anaplastic astrocytoma without an IDH mutation (IDH wild type) have a less favorable prognosis than patients with glioblastoma with an IDH mutation [1]. IDH-mutated glioblastomas are thought to have arisen from grade II or grade III tumors in terms of malignant progression (secondary glioblastomas), in contrast to primary glioblastomas, which are characterized by IDH wild-type and likely represent a biologically different entity.
In the following, we will provide an overview of current therapeutic options taking into account the WHO classification, molecular markers, as well as the age of the patients; a suggested clinical decision pathway is summarized in Figures 1 and 2 .


Anaplastic oligodendrogliomas and oligoastrogliomas WHO grade III
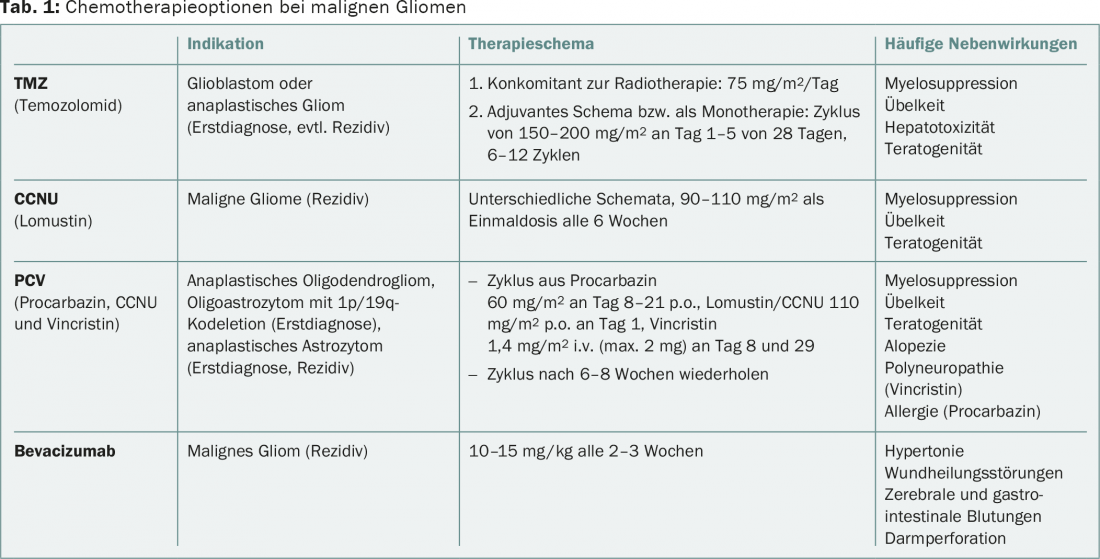
The presence of a 1p/19q codeletion, strictly associated with the IDH mutation, is crucial for the therapeutic decision in anaplastic gliomas of oligodendroglial origin. These patients benefit from a combination of radiotherapy and chemotherapy with procarbazine, lomustine (CCNU), and vincristine (PCV regimen) at initial diagnosis compared with radiotherapy alone, according to long-term follow-up data from the RTOG-9402 and EORTC-26951 trials [2,3]. Data from the NOA-04 trial show equal efficacy of regimens with PCV resp. With temozolomide (TMZ) in anaplastic gliomas overall. Due to the toxicity of the PCV regimen, the better tolerated TMZ is often preferred. That alkylating chemotherapy alone is equivalent to the direct combination of chemotherapy and radiotherapy as first-line therapy is unlikely when considering the available data. In the case of chemotherapy alone at initial diagnosis, radiotherapy should be given if tumor progression occurs. Furthermore, there are no standardized treatment recommendations for tumor recurrence. The options for glioblastoma may also be applied (Table 1).
Anaplastic astrocytomas, WHO grade III
According to the NOA-04 trial, for first-line therapy, chemotherapy with TMZ or PCV followed by radiotherapy at relapse is equivalent to primary radiotherapy followed by TMZ or PCV at relapse. For patients with anaplastic glioma without 1p/19q codeletion, a phase III trial is currently evaluating the role of chemotherapy with TMZ in addition to radiotherapy in first-line treatment (CATNON trial). 1p/19q codeletion, MGMT promoter methylation, and IDH mutation are prognostic biomarkers and may be useful for differentiation from oligodendroglial tumors (1p/19q) as well as glioblastomas (IDH). Furthermore, in anaplastic gliomas without IDH mutation, a methylated MGMT promoter is predictive of prolonged progression-free survival in the case of alkylating chemotherapy with or without radiotherapy. For tumor recurrence after radiotherapy and chemotherapy with TMZ or PCV, there are no firm recommendations; the options are analogous to those for glioblastoma recurrence.
Glioblastoma, WHO grade IV
Prognostically unfavorable features of glioblastomas include advanced age, poor Karnofsky perfomance status, low resection extent, lack of IDH mutation, and an unmethylated MGMT promoter. Radiation therapy with concomitant and adjuvant chemotherapy with TMZ, which has been established since the EORTC-NCIC trial, continues to be considered first-line therapy [4]. MGMT promoter methylation is also predictive of a good response to radiochemotherapy. For elderly patients over 65 or 70 years of age without stratification by molecular profile, no clinically relevant benefit of combined radiochemotherapy has been demonstrated to date, given the high toxicity of the regimen in elderly patients. For elderly patients with methylated MGMT promoter, a survival benefit has been shown with monotherapy with TMZ compared with radiotherapy [5,6]. Without knowledge of MGMT status or in the case of unmethylated MGMT promoter, the treatment of choice in elderly patients is radiotherapy alone with hypofractionated protocol (10× 3.4 Gy), which is equivalent or possibly superior to conventionally fractionated radiotherapy [6].
Bevacizumab, an antibody against vascular endothelial growth factor (VEGF), showed no effect on overall survival in two randomized phase III trials [7,8].
The results of the Phase III EF-14 study were presented at the end of 2014. It showed a survival benefit of 19.6 vs. 16.6 months with the addition of NovoTTF-100A (Tumor Treating Fields) to standard therapy [9]. The therapy consists of alternating electric fields applied through a mask to the head for at least 18 hours a day. The value of NovoTTF for the first-line treatment of glioblastoma in clinical practice is currently being discussed in expert circles as well as by regulatory authorities.
In the case of tumor progression, treatment options are limited, nonstandardized, and poorly evidence-based. A new resection may be reasonable in young patients, good general condition and favorable tumor location. Chemotherapies used include nitrosoureas such as lomustine/CCNU, again TMZ, or bevacizumab (Table 1). Further information can be found in the guidelines of the European Association of Neuro-Oncology (EANO) [10].
Supportive therapies
For the management of gliomas, in addition to tumor-specific therapy, the optimal use of corticosteroids, anticonvulsant medications, and psycho-oncological support is very important. Steroids (dexamethasone up to a maximum of 16 mg as a single dose in the morning) may be useful in cases of clinical deterioration or intracranial pressure and during radiotherapy. To avoid side effects, a time-limited use depending on the clinic should be aimed for. For anticonvulsant therapy, nonenzyme-inducing agents such as levetiracetam or lamotrigine are preferred. Also highly potent in seizure prophylaxis is the enzyme-inhibiting valproic acid, for which direct antitumor effects are also discussed.
Outlook
In current clinical trials, immunotherapeutic approaches are particularly promising. Vaccination studies use vaccines with tumor-specific targets, for example the variant of the EGFR receptor EGFRvIII (ACT-IV study, phase III) or with combinations of tumor-associated antigens (ICT-107 phase II study), or cell-based approaches, including activated dendritic cells. Among immunomodulatory therapies, inhibitors of the programmed death-1 pathway or ligand are most notable. Despite advances in understanding the molecular biology of glioblastoma, further development of successful translational therapies is needed to improve the still limited therapeutic options.
Literature:
- Hartmann C, et al: Long-term survival in primary glioblastoma with versus without isocitrate dehydrogenase mutations. Clin Cancer Res 2013; 19(18): 5146-5157.
- van den Bent MJ, et al: Adjuvant Procarbazine, Lomustine, and Vincristine Chemotherapy in Newly Diagnosed Anaplastic Oligodendroglioma: Long-Term Follow-Up of EORTC Brain Tumor Group Study 26951. Journal of Clinical Oncology 2013; 31(3): 344-350.
- Cairncross G, et al: Phase III trial of chemoradiotherapy for anaplastic oligodendroglioma: long-term results of RTOG 9402. J Clin Oncol 2013; 31(3): 337-343.
- Stupp R, et al: Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 2005; 352(10): 987-996.
- Wick W, et al: Temozolomide chemotherapy alone versus radiotherapy alone for malignant astrocytoma in the elderly: the NOA-08 randomised, phase 3 trial. Lancet Oncol 2012; 13(7): 707-715.
- Malmström A, et al: Temozolomide versus standard 6-week radiotherapy versus hypofractionated radiotherapy in patients older than 60 years with glioblastoma: the Nordic randomised, phase 3 trial. Lancet Oncol 2012; 13(9): 916-926.
- Chinot OL, et al: Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med 2014; 370(8): 709-722.
- Gilbert MR, et al: A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med 2014; 370(8): 699-708.
- Stupp R, et al: NT-40 Interim Analysis of the EF-14 Trial: A Prospective, Multi-center Trial of NovoTTF-100A Together With Temozolomide Compared to Temozolomide Alone in Patients with Newly Diagnosed GBM. Neuro-Oncology 2014; 16 (suppl 5): v167.
- Weller M, et al: EANO guideline for the diagnosis and treatment of anaplastic gliomas and glioblastoma. Lancet Oncol 2014; 15(9): e395-403.
InFo ONCOLOGY & HEMATOLOGY 2015; 3(3-4): 24-27.