Over the last decade, immunotherapy using checkpoint inhibitors has become established in the treatment of many oncological conditions. Especially in advanced tumor disease, ipilimumab, nivolumab and co. play a significant role today. At a virtual media conference, experts reviewed the success story to date and discussed the potential of checkpoint inhibitors in early-stage disease.
Checkpoint inhibitors have become indispensable in the treatment of malignant melanoma and non-small cell lung cancer (NSCLC). However, significant progress has also been made in other entities over the past decade through the use of these immunotherapeutics. Recent data presented at the ESMO(European Society for Medical Oncology) congress show that checkpoint inhibitors are able to prolong survival in advanced disease stages, including renal cell carcinoma and malignant pleural mesothelioma. So a lot has happened since the first approval of a compound targeting CTLA4 in 2011. Patients with NSCLC and malignant melanoma are not the only ones benefiting today from new therapeutic standards based on agents directed against PD1, PD-L1 and CTLA4.
However, its use in earlier, non-metastatic disease stages is still far more restrained. This could soon change in view of current developments in the disease concept and the first promising study data. For example, adjuvant administration of nivolumab in carcinoma of the esophagus and gastroesophageal junction appears to significantly reduce the recurrence rate – a finding that has already led to the Swiss approval of OPTIVO® in this indication. Earlier use of checkpoint inhibitors is promising due to their mechanism of action. The immune system can thus be activated against the tumor as long as it is as functional as possible and has not yet been modified by the disease or other therapies. Immunotherapy in the adjuvant, neoadjuvant, or perioperative setting also offers the opportunity to strike as long as the tumor burden is low – and the chance of cure correspondingly higher. It will probably be decades before the optimal use of the various checkpoint inhibitors in the different tumor entities is clarified. But the groundwork has been laid and patients are already benefiting from earlier and earlier checkpoint inhibitor administration.
|
Participants of the media conference
|
Checkpoint inhibitors in advanced disease stages: new long-term data
New long-term data on dual immunotherapy using ipilimumab plus nivolumab for advanced renal cell carcinoma and malignant pleural mesothelioma were presented at ESMO Congress 2021. These underscore the utility of checkpoint inhibitors in both indications and are another piece of the puzzle in the exploration of the drug class; corresponding approvals have already been obtained.
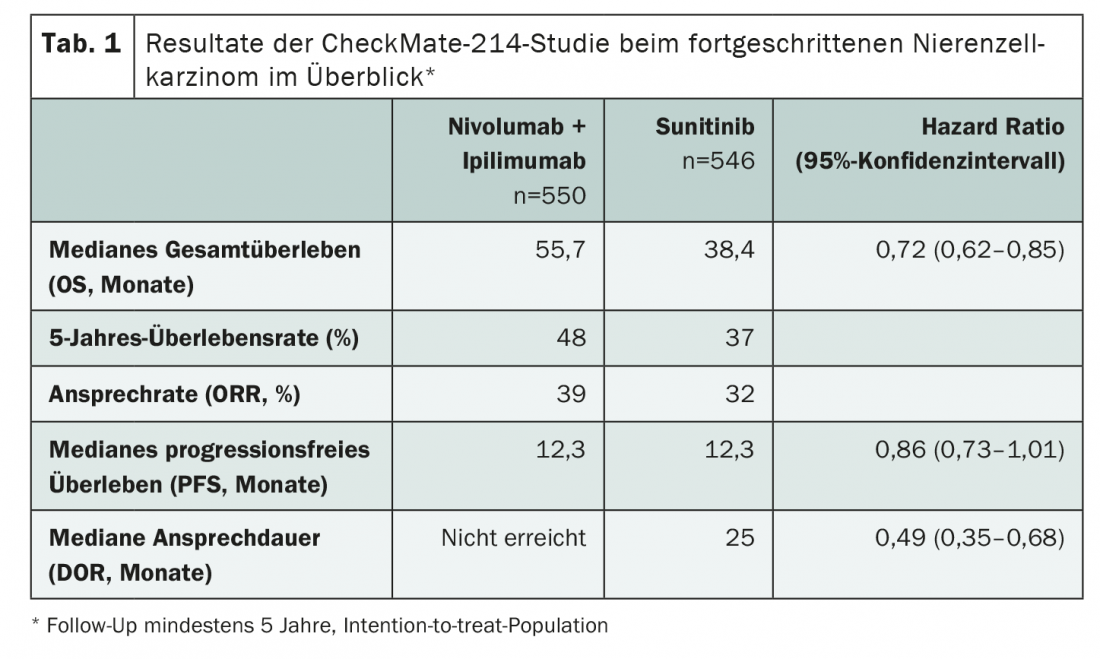
After five years of follow-up, the phase III CheckMate-214 study showed a sustained benefit of nivolumab plus ipilimumab versus sunitinib in first-line advanced renal cell carcinoma (Table 1). In particular, low- and intermediate-risk patients benefited from immunotherapy, with a positive effect observed in all subgroups. Five years is the longest follow-up time to date for a phase III trial using a checkpoint inhibitor combination. With a median overall survival (mOS) of nearly 56 months in the intervention group, the longest overall survival ever observed in a phase III trial in advanced renal cell carcinoma was reported. Also, after five years, 31% of patients on combination therapy with ipilimumab and nivolumab are still without progression. These are clear advances in the treatment of a difficult-to-treat and prognostically unfavorable disease.
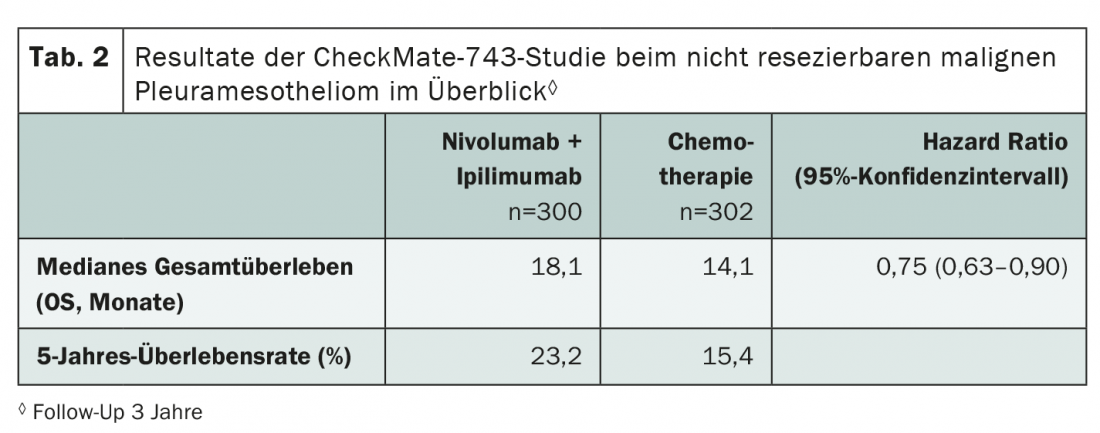
Malignant pleural mesothelioma also represents a prognostically extremely unfavorable clinical picture with so far limited therapeutic options. 3-year data from the CheckMate-743 study now show: survival can be sustainably prolonged by the use of ipilimumab plus nivolumab (Tab. 2) . The dual immunotherapy was compared in the phase III trial with standard chemotherapy (cisplatin/carboplatin plus pemetrexed) in the first line. It is encouraging to see large-scale trials being conducted in rare entities as well – because checkpoint inhibitors have potential here as well, which needs to be further characterized.
Focus on early disease stages: untapped potential?
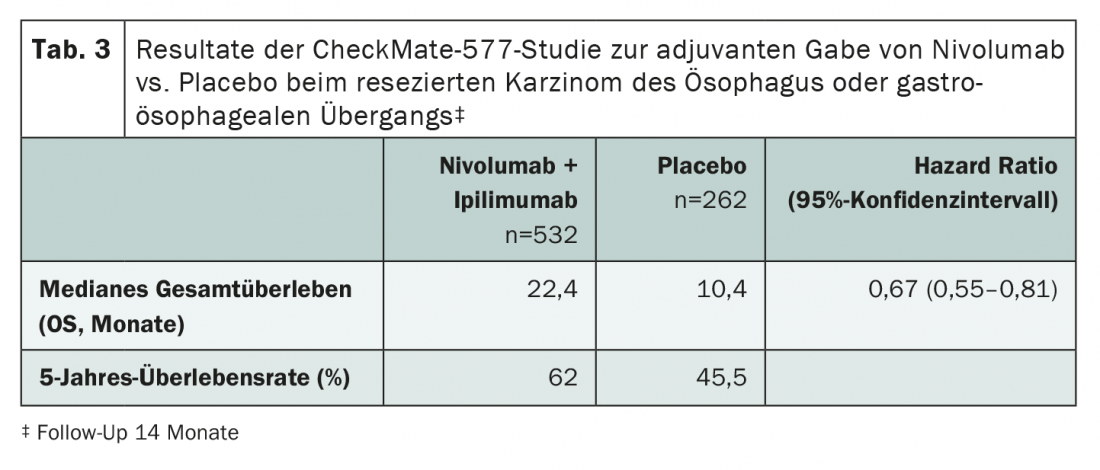
While immunotherapy for advanced cancers is now considered standard of care in many places, its use in earlier stages has been less studied – and thus more controversial. In the interest of optimal disease control, the use of checkpoint inhibitors should also be carefully considered in the adjuvant, neoadjuvant, and perioperative settings. Although individual, sometimes encouraging study results are available here (Tab. 3), longer-term data and studies on first-line treatment are lacking.
There is also a considerable unmet need for the development of new therapies in less advanced cancers. Often, the risk of recurrence is high even after complete resection. For example, this is up to 85% for malignant melanoma, about 70% for hepatocellular carcinoma, and up to 50% for muscle-invasive bladder carcinoma. The use of immunotherapeutic agents to reduce this risk of recurrence makes scientific sense and is currently being investigated in many entities. On the other hand, the additional toxicity must always be considered, which tends to be higher with an intact immune system – as well as the efficacy.
To assess current perceptions and practical implementation of checkpoint inhibitor therapies in early-stage disease, Kald Abdallah, MD, Director of Medical Oncology at Bristol Myers Squibb, presented a recent survey. Responses from 256 oncologists, surgeons, and other specialized physicians from five countries were analyzed. The results indicate an increasing importance of immunotherapy also in the neoadjuvant, adjuvant and perioperative setting. Currently, application is often in the context of studies, indicating a pronounced dynamic in the field. Nevertheless, chemotherapies are still used much more frequently according to the previous standard. Physicians cited long-term survival, prevention of recurrence, and quality of life as the most important factors contributing to treatment decisions, in descending order. Overall, about 90% of respondents showed much enthusiasm for the use of checkpoint inhibitors in earlier stages of disease. The greatest potential was estimated for lung cancer, melanoma and bladder cancer.
The bottom line is that ipilimumab, nivolumab and co. represent an important field of research in increasingly earlier disease stages of diverse oncological syndromes. A number of approvals are likely to be forthcoming in the next few years with the current intensive research efforts. The goal is to identify those patients who will benefit from adjuvant, neoadjuvant or perioperative administration – not forgetting those with rare cancers.
Source: Bristol Myers Squibb ESMO 2021 Media Event: What is the Potential of Immunotherapy to Live Up to the Promise of Long-Term Survival in Earlier Stages of Cancer? Sept. 20, 2021, virtual implementation.
InFo ONCOLOGY & HEMATOLOGY 2021; 9(5): 40-41.