Active surveillance is the preferred option postoperatively for stage I seminoma. In contrast, for non-seminoma stage I, adjuvant chemotherapy with one cycle of BEP is recommended (with risk factor of lymphovascular invasion). Risk-based follow-up plans are used in aftercare.
Testicular carcinoma is the most common malignancy in men aged 20 to 40 years. In total, approximately 450 men per year develop a testicular tumor in Switzerland, which means that testicular tumors account for only 1% of all malignant diseases. More than two-thirds of testicular tumors are diagnosed at an early stage (stage I). Prognosis is excellent with a cancer-specific 5-year survival of >95%.
Postoperative management of stage I seminoma.
It remains controversial whether prognostic risk factors can be defined in stage I seminoma. Several evaluations addressed this question, and the various risk factors for recurrence after orchiectomy without adjuvant treatment were recently summarized in a meta-analysis. Primary tumor size without cut-off value, i.e. as a continuous parameter, is the best validated risk factor; with each centimeter increase in primary tumor size, the risk of recurrence also increases. In contrast, the role of stromal invasion of the rete testis is less well established.
The discussion regarding adjuvant therapy in stage I seminoma has been revisited in recent years due to new evidence:
- Active surveillance is a good option in patients without risk factors with an acceptable low risk of relapse.
- For patients with risk factors, one cycle of carboplatin AUC7 could possibly provide suboptimal protection against recurrence.
A recent evaluation of the Swedish-Norwegian Testicular Tumor Study Group (SWENOTECA) showed a low recurrence rate in patients without risk factors of only 4% (defined as size >4 cm as well as invasion of the rete testis). However, in patients with risk factors, the recurrence rate after single-dose carboplatin was still comparatively high at 9.3%. These data are controversial, as other studies have arrived at lower recurrence rates.
It may currently be stated that patients without risk factors should certainly not receive adjuvant treatment. These patients should receive active surveillance according to published guidelines.
Active surveillance may also be done in patients with risk factors. An alternative is adjuvant chemotherapy with single-dose carboplatin AUC7, although the benefit is controversial.
Adjuvant chemotherapy for stage I non-seminoma with 1× BEP.
Patients with stage I non-seminoma have an indication for adjuvant therapy if risk factors are present. The best documented risk factor is lymphovascular vascular invasion. This risk factor determines the treatment decision. Predominance of embryonal carcinoma on histology is another risk factor for recurrence, although its value is controversial.
Adjuvant therapy for stage I non-seminoma has included two cycles of BEP chemotherapy (bleomycin, etoposide, cisplatin) since the 1990s. The question of whether two cycles of BEP are necessary or whether one cycle would also be sufficient has been addressed in several small prospective studies as well as retrospective evaluations. A randomized trial compared retroperitoneal lymphadenectomy with one cycle of BEP in patients with or without risk factors. There was an excellent outcome in the BEP group with 99% progression-free survival at two years. Data from 571 patients in SWENOTECA with one cycle of BEP and a follow-up of nearly eight years show a recurrence rate of 3.2% in tumors with vascular invasion and 1.6% without vascular invasion, respectively, confirming the long-term effectiveness of therapy with one cycle of BEP.
Thus, it can be stated that in stage I non-seminoma with the presence of lymphovascular vascular invasion, adjuvant chemotherapy should be given with only one cycle of BEP. In the absence of risk factors, active monitoring is the management of choice.
Consistent reduction of chemotherapy exposure is important because the cumulative dose of applied drugs has implications for early and long-term toxicity. Cisplatin causes an increased risk of renal function impairment, hearing loss, neuropathies, and cardiovascular disease. Etoposide exposure is associated with leukemias and myelodysplastic syndromes, and this effect is primarily associated with high-dose therapies. Bleomycin can lead to pulmonary toxicity including. Lead to pulmonary fibrosis.
Value of FDG-PET-CT examination in testicular tumors.
The question of whether FDG-PET-CT should be used in men with germ cell tumors of the testis has been raised repeatedly. The guidelines here agree that PET-CT examination has basically no value in testicular tumor – PET-CT should not be routinely used in regular staging, primary response monitoring, or follow-up. The reasons for this are that PET-CT has low sensitivity and specificity in testicular tumors and thus has no advantage over computed tomography. Thus, PET-CT examination does not lead to any information gain and has no influence on treatment decisions.
The only exception to this rule is the situation of the patient with metastatic seminoma who is found to have a large residual tumor after completion of chemotherapy. It is important to note that the following comments refer exclusively to seminoma; in non-seminoma, any residual tumor of >1 cm in size must be resected. PET-CT cannot provide any assistance in non-seminoma, since mature teratoma is FDG negative and thus in up to 30% of cases a false-negative result of PET-CT must be assumed.
What about PET-CT in the patient with seminoma and residual tumor after chemotherapy? First, CT should be performed at the completion of chemotherapy to monitor response. The further procedure then depends on the size of the residual tumor: Various studies have shown that residual tumors in seminoma exclusively contain necrosis if they are <3 cm in size. In this case, no further examinations or therapies are necessary and the patient can be submitted to the usual follow-up care according to Swiss recommendations. However, if the residual tumor is >3 cm, vital tumor tissue could still be present in addition to necrosis. In this setting, PET-CT can be performed, but no earlier than eight to 10 weeks after completion of chemotherapy to minimize the risk of false-positive results. The negative predictive value of PET-CT is high (>90%) and in case of a negative PET-CT the patient is allowed to follow up. If the PET-CT result is positive, the further procedure is unclear. Recent data suggest that the positive predictive power of PET-CT is very limited and only about 25%. In this situation, it is therefore recommended to perform regular follow-up CT scans and to start further therapy only if there are clear indications of progression. In principle, patients with PET-positive residual tumors should be discussed with a specialized center before initiating treatment.
Therapy of the recurrence
It is important to distinguish different clinical scenarios of recurrence. In the case of recurrence under Active Surveillance or after adjuvant chemotherapy or radiotherapy, treatment is the same as if de novo disease were present.
This must be distinguished from recurrence after therapy with three to four cycles of cisplatin-containing chemotherapy. In the following, only this clinical situation will be discussed.
It remains unclear how best to treat these patients. Lorch et al. have retrospectively analyzed over 1500 cases and defined prognostic risk factors and a risk group classification. In a second analysis of this cohort, survival was looked at as a function of the treatment chosen. Retrospectively, there was an advantage for the use of high-dose therapy followed by autologous stem cell transfusion for all risk groups. However, other data show that conventional salvage chemotherapy can often be successful and that high-dose treatment should only be performed in a later line. To clarify this important question, an international randomized trial (TIGER trial: TIP chemotherapy vs. dual high-dose therapy) is currently being conducted – also in Switzerland (University Hospitals of Zurich, Bern, Geneva). Since recurrence after curatively intended chemotherapy is very rare (about 20 cases per year in Switzerland), these patients should be assigned to one of the study centers. This ensures optimal treatment for all Swiss men with recurrence. Salvage therapy for germ cell tumor of the testis should never be started without consulting a specialized center.
Follow-up recommendations
In 2010, recommendations on follow-up were published by an interdisciplinary Swiss group. These recommendations were also adopted by the German testicular tumor group. On the occasion of the ESMO Consensus Conference 2016, the recommendations were again simplified and adapted and have now been adopted by both oncologists (ESMO) and urologists (EAU).
The basis of follow-up is to adjust the frequency of visits and modality of imaging to the recurrence frequency and pattern. Imaging should be used with restraint and ionizing radiation using CT should be avoided if possible. In principle, a CT thorax is not necessary during follow-up; the abdomen should be checked by MRI.
Basically, three groups are to be distinguished in the aftercare:
- Seminoma stage I (regardless of therapy chosen)
- Non-Seminoma Stage I with Active Surveillance
- All patients with adjuvant or curative intended therapy with achievement of complete remission.
The current follow-up recommendations for the three groups are shown in Tables 1-3.

It is important to emphasize that these recommendations apply only to patients who are in complete remission (for non-seminoma, after chemo and possible surgery; for seminoma, either residual findings <3 cm or negative PET if >3 cm). If a “poor prognosis” situation is present at the start of therapy, individualized follow-up should be provided.
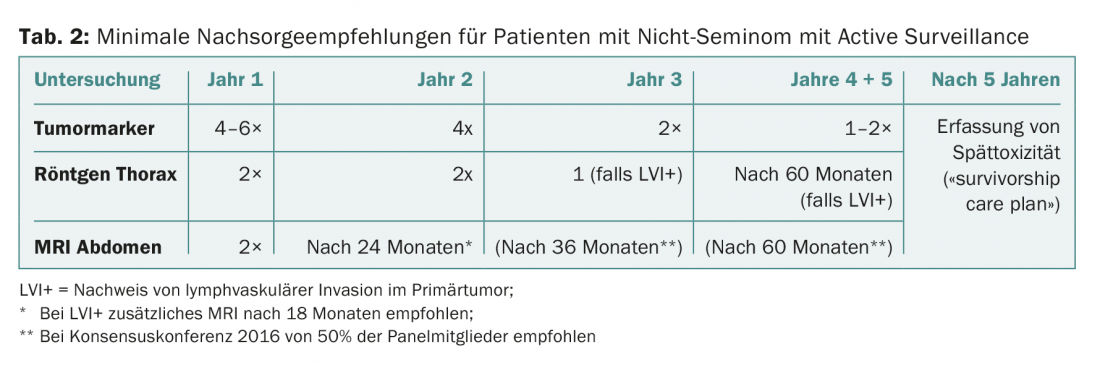
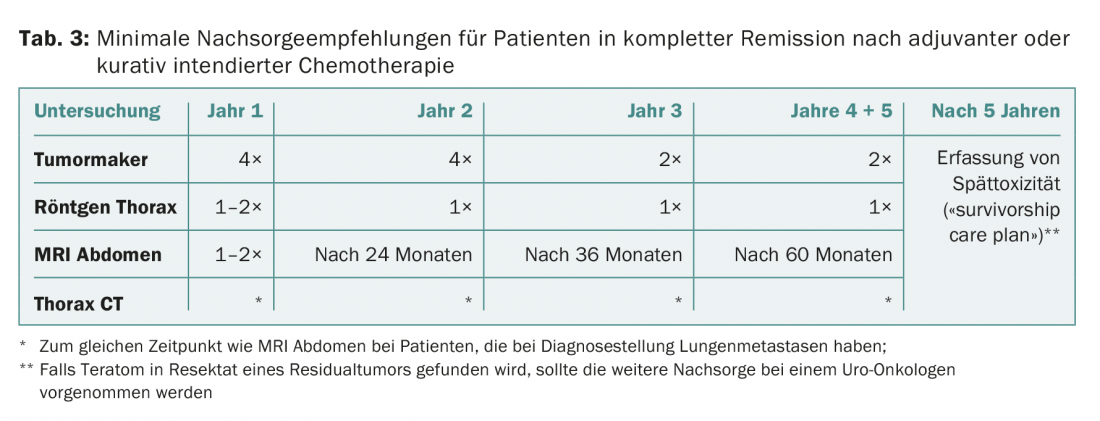
After five years, the risk of recurrence is extremely small and is <0.5%. In principle, therefore, no determinations of tumor markers and no regular imaging are required after five years. The focus is on early detection and treatment of late toxicities. Once a year, monitoring of cardiovascular risk factors, renal function, and gonadal function is recommended. In addition, the men should be motivated to adhere to a healthy lifestyle (no nicotine, sufficient exercise, not overweight, balanced diet, little alcohol).
Take-Home Messages
- In stage I seminoma, active surveillance can be recommended as the preferred option in the majority of patients. Patients with risk factors for recurrence should be counseled regarding adjuvant therapy with one cycle of carboplatin AUC7.
- In stage I non-seminoma, adjuvant chemotherapy with one cycle of BEP should be given to patients with the risk factor of lymphovascular invasion.
- FDG-PET-CT should be used only for residual findings >3 cm in patients with metastatic seminoma after chemotherapy.
- The indication for high-dose therapy in case of recurrence depends on a number of factors. Patients with recurrences should be treated in specialized centers.
- Risk-based follow-up plans should be used in the follow-up of patients with testicular tumors.
InFo ONCOLOGY & HEMATOLOGY 2018; 6(1): 11-14.