Approximately half of patients with newly diagnosed NSCLC can be treated with curative therapy. In the first three years thereafter, the likelihood of relapse is greatest. There is no good evidence for intensive follow-up. Especially not for close radiological follow-up.
Lung cancer is the most common tumor disease leading to death. The poor prognosis is related in part to the fact that only about 50-55% of patients with non-small cell lung cancer (NSCLC) are diagnosed at a stage where potentially curative therapy can be offered (stages I-III). Depending on the stage, 30-75% of curatively operated patients suffer a recurrence [1–3]. Most relapses occur in the first three years postoperatively [1,4]. The Ludwig Lung Study Group investigated the pattern of relapse in three prospective studies [4]. Of 426 patients with documented relapse, only 26% had local recurrence. Median survival after relapse was less than one year [5]. Furthermore, patients after curatively treated NSCLC are at high risk for the occurrence of second tumors, predominantly recurrent bronchial carcinomas, head and neck tumors, and esophageal carcinomas [6]. Metachronous pulmonary second cancers occur at an incidence of 1-9% per year [7–9]. In metachronous second tumors of the lung, there is a chance of a renewed, potentially curative therapeutic approach (surgery or stereotactic radiation).
The main purpose of follow-up in patients with curatively treated NSCLC is to detect recurrence in a situation where renewed curative therapy may improve overall survival and significantly improve quality of life. This thus includes both the detection of local recurrence amenable to further curative local ablative therapy (surgery or stereotactic irradiation) and the detection of solitary distant metastases (especially lung, brain, adrenal glands), which in selected patients can also be resected with curative intent or irradiated stereotactically. There are no prospective randomized trials demonstrating the value of structured radiologic follow-up in terms of overall survival. In this review article, we would like to discuss the evidence for follow-up and the recommendations of various professional societies.
Evidence on follow-up in bronchial carcinoma.
Prospective randomized trials: Moore et al. randomized 203 patients between conventional follow-up and follow-up by nurses specialized in lung cancer [10]. All patients had completed their curative treatment and had a life expectancy of at least three months. Conventional follow-up included outpatient visits every two to three months. The intervention group had monthly contact with a specialist nurse, either by telephone or at a consultation to identify symptoms of disease progression or complications of previous therapy. Patients in the intervention group had statistically significant less dyspnea, lower rates of peripheral neuropathy, and better quality of life at 12 months. The intervention group also performed significantly better in terms of satisfaction at three, six, and twelve months. Median survival was the same in both groups, but the median time to symptomatic progression was shorter in the intervention group (6 vs. 10.2 months). This study shows that more intensive and structured patient contact during follow-up is important for patient satisfaction and quality of life and leads to earlier detection of recurrence, but does not change prognosis.
Retrospective comparative studies: Virgo et al. analyzed 182 patients with resected NSCLC [11]. Patients were divided into an intensive follow-up group (n=120) and a less intensive follow-up group (n=62). Even though the analysis was retrospective, the groups were largely balanced. However, patients with more intensive follow-up protocols had significantly more comorbidities. In this study, more intensive follow-up-characterized by the frequency and extent of laboratory chemistry and radiologic monitoring-did not show improvement in the time to detection of recurrence, metastases, or second tumors. Overall survival was also not different in the two groups.
Nakamura et al. Retrospectively studied 1398 patients who were followed up by either thoracic surgeons (n=846) or pulmonologists (n=552) after curative resection of NSCLC [12]. Follow-up by thoracic surgeons included a clinical examination and chest radiograph one month after surgery and then every three to four months for three years. Pulmonologists subjected patients to CT thorax every six months. Patients in thoracic surgery follow-up had increased NSCLC-associated mortality (hazard radio, HR 1.279). Because of the heterogeneity of the groups, especially in the radiological examinations, these results should be interpreted with caution.
Younes and Gross retrospectively compared intensive structured follow-up (n=67) with symptom-based follow-up (n=63) in patients after curatively resected NSCLC [13]. Intensive follow-up included clinical examinations (weeks 1, 3, and 8, then bimonthly for the first six months and trimonthly until two years postoperatively), chest radiographs (weeks 1 and 3 and then at 2, 4, 9, 15, and 24 months), and liver function tests (at one and two years). Clinical characteristics of the two groups were comparable. Neither disease-free nor overall survival was significantly different. Most patients were diagnosed with recurrence or metastasis based on clinical symptoms.
Single-arm studies: In a prospective study at the University Hospital Basel, 563 patients were followed up for ten years after curative resection of NSCLC using clinical controls and chest X-ray examinations [14]. The follow-up interval was three-monthly for two years, six-monthly for an additional three years, and annually in years 6-10. This follow-up allowed 3.1% of patients to pursue another curative intent treatment option. Approximately one-third of patients with recurrence were diagnosed based on symptoms and not during structured follow-up. This study also examined the cost of structured follow-up. They were stated at 90,000 Swiss francs per year of life gained.
Walsh et al. were able to show in a cohort of 358 patients that early diagnosis of recurrence in asymptomatic patients did not improve prognosis [15]. Westeel et al. presented data from an intensive follow-up program with bronchoscopy and computed tomography of the lungs, liver, and adrenal glands every six months [16]. In 192 curatively resected NSCLC patients, the recurrence rate was 71%. Recurrence was detected by structured follow-up in 26% of patients. Survival at three years was 31% in asymptomatic patients.
An Asian study examined structured follow-up with CT [17]. 986 patients with resected NSCLC (48% stage I) were retrospectively evaluated. Patients were followed up at three-month intervals for the first two years and then at four-month intervals until five years after surgery. Contrast-enhanced CT scans were performed every three months for two years and then every six months. In addition, PET/CT was performed one year after surgery and if recurrence was suspected. In this study, the rate of symptomatic recurrence was low at 18%. 50% of recurrences were locoregionally confined and 39% of patients underwent a second curative resection. The median survival after relapse was 43.6 months. These very good results have to be interpreted with caution, especially since this is an Asian population and accordingly many non-smokers were included who have a different tumor biology (e.g. more EGFR mutations) and also a smaller risk for second cancers.
Hanna et al. investigated the value of minimal-dose CT (MnDCT) in 271 patients after resection of NSCLC [18]. Patients were followed up with MnDCT and conventional chest radiography at months 3, 6, 12, 18, 24, 36, 48, and 60 after surgery. Suspicious findings were further clarified with conventional CT and biopsies. MnDCT was more sensitive (94% vs. 21%, p<0.0001) and had higher negative predictive value (99% vs. 96%, p=0.007). Overall, 23.2% of patients were diagnosed with recurrence, with 78% of patients being asymptomatic. The median survival in 37 patients who underwent further curative therapy was 69 months. These are excellent results in a selected group of patients, with no comparison to a group with less intensive tumor follow-up.
Evidence on the use of PET/CT in follow-up.
The main problem with the use of PET/CT in follow-up is postoperative inflammatory changes in the thorax, which can lead to false-positive findings, because such changes are often accompanied by strong FDG enhancement. Such inflammatory changes may still be present six months postoperatively [19]. The rate of false positives at this early stage is over 10% [20]. This problem is even more pronounced after local radiotherapy [21,22]. A potential advantage of PET/CT is the early detection of distant metastases. As a caveat, it must be said that the power of PET/CT is limited in the detection of brain metastases, a common site of recurrence. PET/CT also has potential utility in the early detection of second tumors. However, a prospective, nonrandomized study found no advantage of PET/CT over conventional radiologic examinations in NSCLC follow-up. The radiation exposure during PET/CT (approx. 11 mSV) is significantly higher than that of a “low-dose” chest CT (2 mSV) or a conventional chest X-ray (0.05 mSV) [20,23]. In addition, the costs are also significantly higher. In addition, there are the costs for further clarification of false-positive findings. To date, there are no studies showing a benefit of PET/CT in follow-up with respect to survival or quality of life.
Current recommendations of the professional societies
Most professional societies recommend regular clinical controls and conventional radiological imaging of the thorax or CT thorax (Tab. 1). In clinical practice, many physicians do not follow these recommendations and perform additional imaging controls with PET/CT or MRI [24]. Interestingly, in a survey conducted by the Society of Thoracic Surgeons, only a small proportion of physicians believe that their tumor follow-up behavior is likely to provide a survival benefit to patients [25,26].
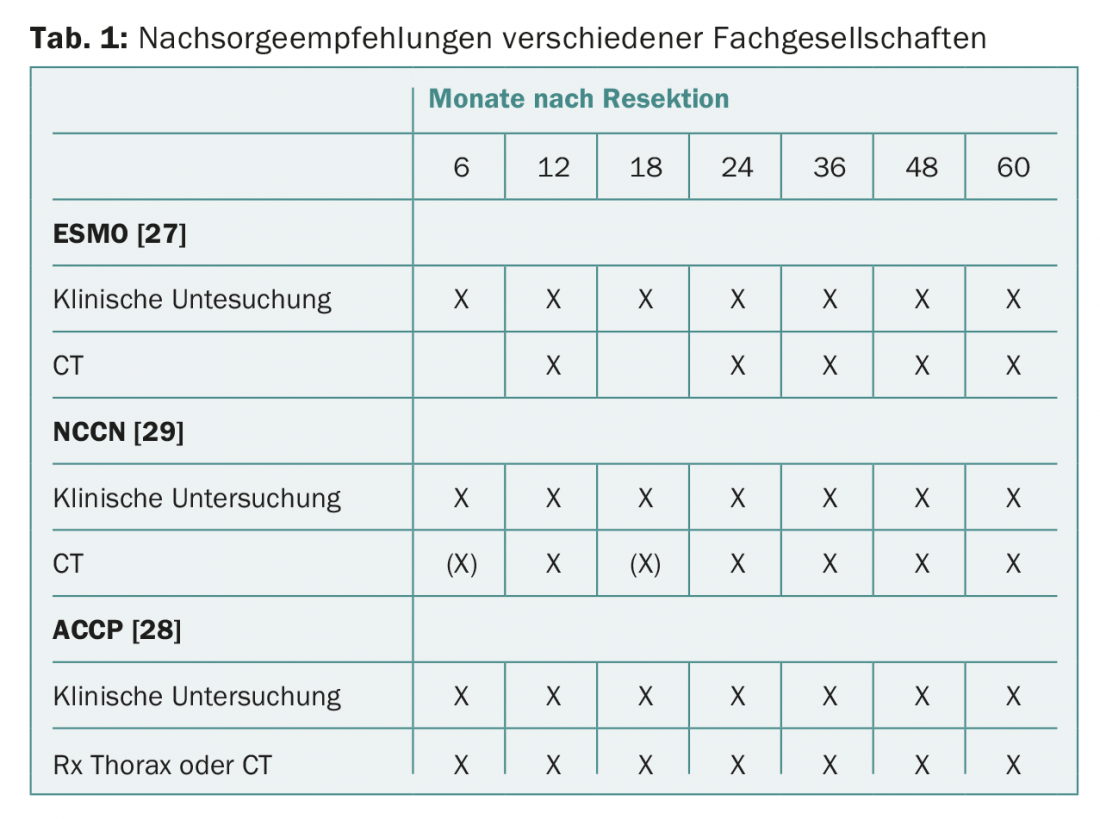
ESMO (European Society for Medical Oncology) guidelines recommend clinical monitoring every three to six months for the first two to three years and annually thereafter. Regarding imaging, chest X-ray and annual computed tomography of the thorax are recommended [27].
The American College of Chest Physicians (ACCP) recommends that patients undergoing curative resection of NSCLC be monitored by chest CT every six months for the first two years and annually thereafter. Performing PET examinations in follow-up is not recommended [28].
The National Comprehensive Cancer Network (NCCN) recommendations for bronchial cancer suggest clinical examinations and chest CT with or without contrast every six to twelve months for two years, then annual CT examinations without contrast [29]. PET or cranial MRI are not recommended.
Summary
Despite the potential benefit of intensive and image-based follow-up for early detection of local recurrences that can be addressed again curatively and early detection of second tumors, there is little evidence to support the routine use of examinations beyond periodic physical examination and conventional chest radiograph. No follow-up study has shown improvement in survival or quality of life in NSCLC patients. Most of these studies are retrospective and have many limitations. Additional radiological examinations are sometimes associated with high costs. Several studies have shown that intensive follow-up is not cost-effective [11,14]. In addition, it should be mentioned here that increased use of sensitive radiological examinations in follow-up increases the risk of false-positive findings, which in turn necessitates additional examinations, which is a burden for patients and generates additional costs.
The majority of NSCLC patients operated on with curative intent are older and have concomitant disease. Many are active or former smokers. These points warrant regular medical consultations. Despite the lack of evidence, most professional societies recommend the use of CT scans of the thorax every three to six months in addition to a clinical examination. There is a high need for prospective studies to evaluate the value of radiologic examinations in follow-up. Outside of study protocols, we recommend that follow-up be limited essentially to clinical controls and conventional chest radiography. It is important to discuss the importance and implementation of follow-up with patients. An important aspect of follow-up care is also to motivate patients to stop smoking. Patients who continue to smoke have an increased risk of relapse, a higher risk of second tumor occurrence, increased noncancer mortality, and poorer overall survival [30–32].
Take-Home Messages
- Only about half of patients with newly diagnosed non-small cell lung cancer (NSCLC) can undergo curative therapy.
- The probability of relapse after curative therapy in NSCLC is 30-75% depending on stage and is greatest in the first three years.
- There is no good evidence for intensive follow-up after curatively treated NSCLC, especially for close radiological follow-up. Most patients can no longer be treated curatively in the event of a recurrence.
- Follow-up should essentially include clinical follow-up, conventional radiographs of the thorax, and CT scans at most.
Literature:
- Martini N, et al: Incidence of local recurrence and second primary tumors in resected stage I lung cancer. J Thorac Cardiovasc Surg 1995; 109: 120-129.
- al-Kattan K, et al: Disease recurrence after resection for stage I lung cancer. Eur J Cardiothorac Surg 1997; 12(3): 380-384.
- Martin J, et al: Long-term results of combined-modality therapy in resectable non-small-cell lung cancer. J Clin Oncol 2002; 20: 1989-1995.
- No authors listed: Patterns of failure in patients with resected stage I and II non-small-cell carcinoma of the lung. The Ludwig Lung Cancer Study Group. Ann Surg 1987; 205: 67-71.
- Sugimura H, et al: Survival after recurrent nonsmall-cell lung cancer after complete pulmonary resection. Ann Thorac Surg 2007; 83: 409-417.
- Thomas P, Rubinstein L: Cancer recurrence after resection: T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg 1990; 49(2): 242-247.
- Johnson BE: Second Lung Cancers in Patients After Treatment for an Initial Lung Cancer. J Natl Cancer Inst 1998; 90: 1335-1345.
- Deschamps C, et al: Multiple primary lung cancers. Results of surgical treatment. J Thorac Cardiovasc Surg 1990; 99: 769-777.
- Martini N, Melamed MR: Multiple primary lung cancers. J Thorac Cardiovasc Surg 1975; 70: 606-612.
- Moore S, et al: Nurse led follow up and conventional medical follow up in management of patients with lung cancer: randomised trial. BMJ 2002; 325: 1145.
- Virgo KS, et al: Post-treatment management options for patients with lung cancer. Ann Surg 1995; 222: 700-710.
- Nakamura R, et al: Postoperative follow-up for patients with non-small cell lung cancer. Oncology 2010; 33: 14-18.
- Younes RN, Gross JL: Follow-up in Lung Cancer How Often and for What Purpose? Chest 1999; 115: 1494-1499.
- Egermann U, et al: Regular follow-up after curative resection of nonsmall cell lung cancer: a real benefit for patients? Eur Respir J 2002; 19: 464-468.
- Walsh GL, et al: Is follow-up of lung cancer patients after resection medically indicated and cost-effective? Ann Thorac Surg 1995; 60: 1563-1570.
- Westeel V, et al: Relevance of an intensive postoperative follow-up after surgery for non-small cell lung cancer. Ann Thorac Surg 2000; 70: 1185-1190.
- Song IH, et al: Prognostic factors for post-recurrence survival in patients with completely resected Stage I non-small-cell lung cancer. Eur J Cardiothorac Surg 2014; 45: 262-267.
- Hanna WC, et al: Minimal-dose computed tomography is superior to chest x-ray for the follow-up and treatment of patients with resected lung cancer. J Thorac Cardiovasc Surg 2014; 147: 30-33.
- Kanzaki R, et al: Clinical value of F18-fluorodeoxyglucose positron emission tomography-computed tomography in patients with non-small cell lung cancer after potentially curative surgery: experience with 241 patients. Interact Cardiovasc Thorac Surg 2010; 10: 1009-1014.
- Choi SH, et al: Positron emission tomography-computed tomography for postoperative surveillance in non-small cell lung cancer. Ann Thorac Surg 2011; 92: 1826-1832; discussion 1832.
- Dahele M, et al: Radiological changes after stereotactic radiotherapy for stage I lung cancer. J Thorac Oncol 2011; 6: 1221-1228.
- Huang K, et al: Radiographic changes after lung stereotactic ablative radiotherapy (SABR) – Can we distinguish recurrence from fibrosis? A systematic review of the literature. Radiother Oncol 2012; 102: 335-342.
- Brix G, et al: Radiation exposure of patients undergoing whole-body dual-modality 18F-FDG PET/CT examinations. J Nucl Med 2005; 46: 608-613.
- Edelman MJ, Schuetz J: Follow-up of local (stage I and stage II) non-small-cell lung cancer after surgical resection. Curr Treat Options Oncol 2002; 3: 67-73.
- Naunheim KS, et al: Clinical surveillance testing after lung cancer operations. Ann Thorac Surg 1995; 60: 1612-1616.
- Virgo KS, et al: Lung cancer patient follow-up: motivation of thoracic surgeons. Chest 1998; 114: 1519-1534.
- Vansteenkiste J, et al: 2nd ESMO Consensus Conference on Lung Cancer: early stage non-small cell lung cancer consensus on diagnosis, treatment and follow-up. Ann Oncol 2014 Aug; 25(8): 1462-1474.
- Colt HG, et al: Follow-up and surveillance of the patient with lung cancer after curative-intent therapy: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013; 143: e437S-54S.
- National Comprehensive Cancer Network: NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®); Non-Small Cell Lung Cancer. Non-Small Cell Lung Cancer (version 5; 2017).
- Warren GW, et al: Smoking at diagnosis and survival in cancer patients. Int J Cancer 2013; 132: 401-410.
- Park SM, et al: Prediagnosis smoking, obesity, insulin resistance, and second primary cancer risk in male cancer survivors: National Health Insurance Corporation Study. J Clin Oncol 2007; 25: 4835-4843.
- Gajdos C, et al: Adverse effects of smoking on postoperative outcomes in cancer patients. Ann Surg Oncol 2012; 19: 1430-1438.
InFo ONCOLOGY & HEMATOLOGY 2017; 5(3): 18-21.