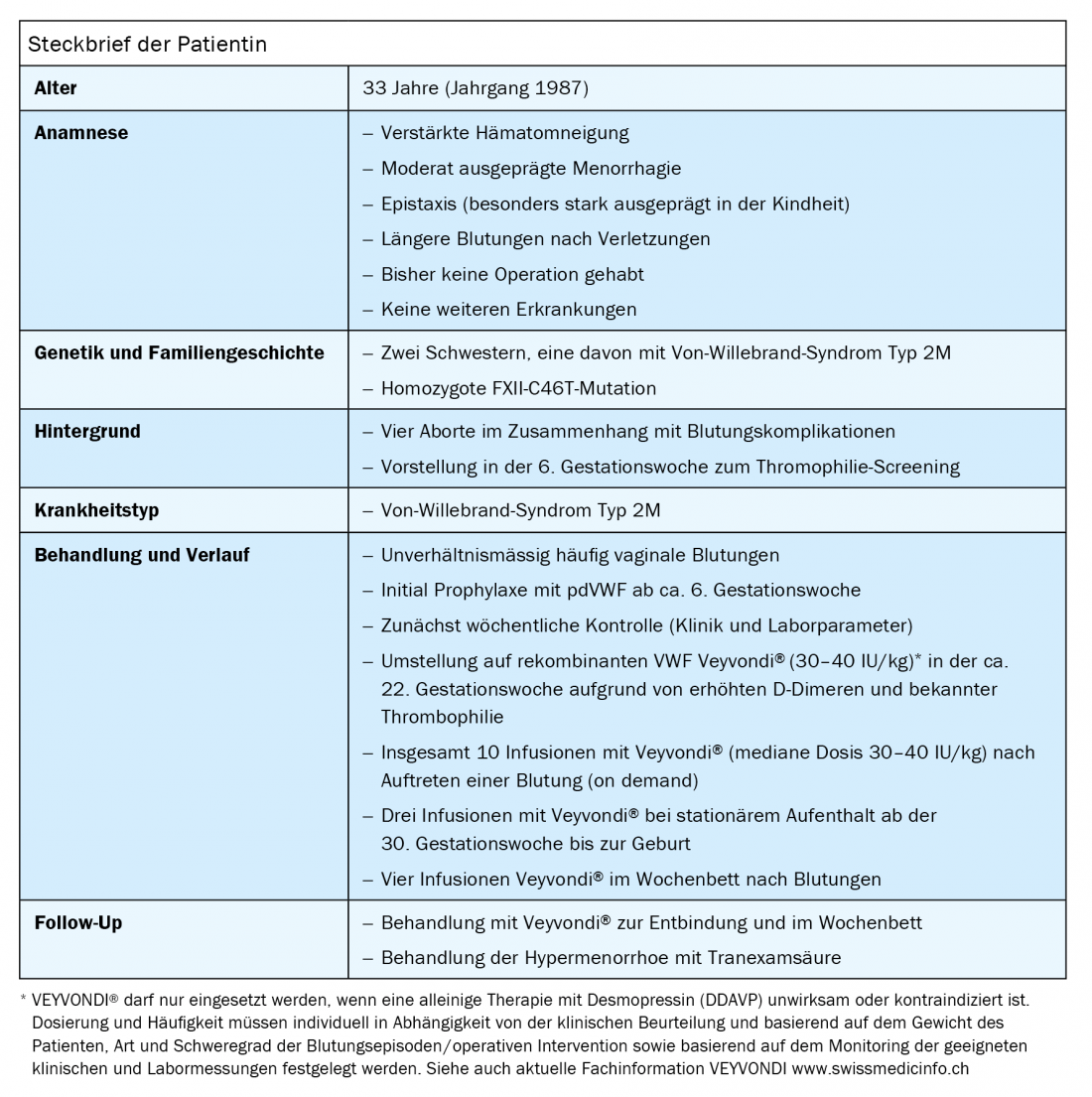
A patient who had already suffered four miscarriages related to bleeding complications presents for thrombophilia screening at the 6th week of gestation. During the diagnostic process, Von Willebrand syndrome is detected, which must be taken into account in the course of pregnancy, during delivery and in the puerperium.
|
Laboratory values
|
Baseline
(10.12.2018)
|
23. SSW
(20.03.2019)
|
29TH SSW
(06.05.2019)
|
|
aPTT
|
26s
|
25s
|
24s
|
|
FVIII:C
|
123%
|
231%
|
238%
|
|
VWF:Ag
|
106.0%
|
185.5%
|
196.8%
|
|
VWF:RCo
|
49.1%
|
52.8%
|
60.9%
|
|
VWF:CBA
|
46.0%
|
53.2%
|
63.7%
|
|
VWF:RCo/VWF:Ag Ratio
|
0.5
|
0.3
|
0.3
|
|
Protein S activity
|
N/A
|
45.3%
|
N/A
|
|
Free protein S
|
N/A
|
51.9%
|
N/A
|
|
D-dimers
|
N/A
|
2.27 mg/L
|
2.67 mg/L
|
|
PFA-100
|
231s/147s
|
167s/110s
|
N/A
|
| Comment by Rosa Sonja Alesci, MD
|

See also current technical information VEYVONDI® www.swissmedicinfo.ch
Abbreviations
aPTT = activated partial thromboplastin time; FVIII:C = factor VIII activity; GI = gastrointestinal; IU = international unit; PFA-100 = Platelet Function Analyzer 100; VWF:Ag = von Willebrand factor antigen; VWF:CBA = von Willebrand factor collagen binding activity; VWF:RCo = von Willebrand factor ristocetin cofactor activity; CNS = central nervous system.

C-APROM/CH//0782 07/2020
VEYVONDI
®
Brief technical information
Z: Active ingredient: Vonicogum alfa. I: Treatment of hemorrhage or bleeding due to surgery in the from–Willebrand–disease when therapy with desmopressin (DDAVP) alone is ineffective or contraindicated. D: Dosage and frequency must be individualized based on clinical judgment and based on patient weight, type and severity of bleeding episodes/surgical intervention, and based on monitoring of appropriate clinical and laboratory measurements; Intravenous administration. AI: Hypersensitivity to the active substance or any of the excipients. Known allergic reaction to mouse– or hamster proteins.
VM:
There are hypersensitivity– or allergic reactions occurred, which may progress to severe anaphylaxis. Monitor patients closely during infusion. Neutralizing antibodies can be formed against Von–Willebrand–Develop factor. There is a risk of thrombotic events. SS: Use only with clear indication. UW: From–Willebrand–Factor Inhibitors, Hypersensitivity– or allergic reactions, tremor, hypertension, vertigo, deep vein thrombosis, ECG changes. IA: There are no known interactions. P: Lyophilisate with 650 I.U. or 1,300 I.U. with 5 ml (resp. 10 ml for 1,300 I.E.) water for injection. Levy category B. Z: Takeda Pharma AG, 8152 Opfikon. You can find detailed information at www.swissmedicinfo.ch


 Von Willebrand syndrome is significantly underdiagnosed overall, as this case of a 33-year-old female patient also shows. Many sufferers have become accustomed to the symptoms and do not see a doctor. Thus, during thrombophilia screening in the context of habitual abortions, it is not uncommon for Von Willebrand syndrome to be diagnosed as an incidental finding. In the present case, especially in view of the four previous miscarriages with hemorrhage and the very impressive bleeding history, replacement of Von Willebrand factor during pregnancy was essential. In addition, the patient had to be closely monitored, especially at the beginning, since was also known to have thrombophilia. This made it possible to respond to individual circumstances and to react quickly to conspicuous complications. Overall, the present case demonstrates that recombinant Von Willebrand factor (Veyvondi®) can be successfully used in a rare type of Von Willebrand syndrome. After a history of four miscarriages, the vaginal bleeding during pregnancy was optimally treated and the birth was also without complications. No thrombotic side effects occurred and Veyvondi® could also be administered without problems in the puerperium.
Von Willebrand syndrome is significantly underdiagnosed overall, as this case of a 33-year-old female patient also shows. Many sufferers have become accustomed to the symptoms and do not see a doctor. Thus, during thrombophilia screening in the context of habitual abortions, it is not uncommon for Von Willebrand syndrome to be diagnosed as an incidental finding. In the present case, especially in view of the four previous miscarriages with hemorrhage and the very impressive bleeding history, replacement of Von Willebrand factor during pregnancy was essential. In addition, the patient had to be closely monitored, especially at the beginning, since was also known to have thrombophilia. This made it possible to respond to individual circumstances and to react quickly to conspicuous complications. Overall, the present case demonstrates that recombinant Von Willebrand factor (Veyvondi®) can be successfully used in a rare type of Von Willebrand syndrome. After a history of four miscarriages, the vaginal bleeding during pregnancy was optimally treated and the birth was also without complications. No thrombotic side effects occurred and Veyvondi® could also be administered without problems in the puerperium.