In case of initial tumor diagnosis during pregnancy, there are established therapeutic procedures for the second and third trimester. If pregnancy occurs after tumor therapy, careful monitoring is required. Impending premature births and fetal growth retardation can thus be detected at an early stage.
In Switzerland, more than 38,500 people are diagnosed with cancer every year. 13% of tumor cases occur before the age of 50, thus mostly still affecting the fertile phase of life. Approximately 190 new cases are registered in children every year. The most common are leukemias (34%), central nervous system tumors (21%), and lymphomas (11%). The chances of cure have improved significantly and now reach 80% [1].
Cytotoxic therapies (radio- and chemotherapies) may temporarily or definitely disrupt testicular and ovarian function. The extent of gonadal damage depends on the type and intensity of treatment as well as individual factors such as biological age. Due to increasing survival rates, more and more patients are facing long-term consequences of chemotherapy or radiotherapy. Since the gonads (ovaries and testes) are particularly sensitive to such exposures, the question of the effects on pregnancy also often arises.
On the other hand, statistics show that parents are significantly older when their first child is born than they were decades ago. We are therefore increasingly encountering the problem that family planning has not yet been completed at the time of cancer diagnosis or treatment. Therefore, the frequency of tumor diagnoses in pregnancy is also increasing, with a prevalence of one to two cases per 1000 pregnancies [2].
Female fertility after oncological therapy
Oncological treatments may include surgical measures, radiotherapy, chemotherapy, endocrinological or immunological procedures, depending on the type of tumor.
Considerations of the influence of oncological therapies on a later desire to have children usually focus on limitations of gonadal function, but effects on the uterus and concomitant maternal diseases must also be taken into account. For example, radiation therapy can cause fibrosis, which prevents timely fetal development until the expected delivery date. Radiation therapy to the abdomen or pelvis can increase the risk of spontaneous abortions 1.5 to 2-fold. In addition to direct damage to the endometrium, fibrosis of the myometrium or the supplying blood vessels is discussed as a possible cause [3].
Tumor therapy can also reduce the resilience of the maternal organism in a later pregnancy, for example by decreasing the function of the heart, kidneys or lungs. For example, if anthracycline therapy leads to cardiomyopathy, maternal circulatory function is impaired during pregnancy [4].
If the ovaries, uterus or testes are affected by tumors, surgical removal of the organs inevitably leads to loss of fertility. In these cases, only egg or sperm donation, surrogacy or adoption can fulfill the desire to have a child.
Chemotherapy or radiotherapy may affect the gonads by destroying primordial follicles in the ovaries or spermatogonia in the testes. The toxicity of chemotherapy is determined by the active ingredient, dose, route of administration, and combination. The extent of gonadotoxicity is related to disease, age, sex, and fertility before tumor therapy. Damage to the hypothalamic-pituitary axis is also possible. The local effect of radiotherapy depends on the irradiation field, radiation dose and fractionation.
Endocrine treatments – as often used for breast carcinoma – have an impact on ovarian function and endometrium [5].
To date, there is little data on the effects of immunotherapy on fertility [6].
Cytostatic drugs have varying degrees of harm to the gonads. If family planning has not been completed, highly gonadotoxic cytostatic drugs (Table 1) should be avoided. Cytostatic drugs with a low risk of ovarian damage include methotrexate, 5-fluorouracil, vincristine, vinblastine, bleomycin, and actinomycin. The risk of permanent amenorrhea varies widely with different regimens.
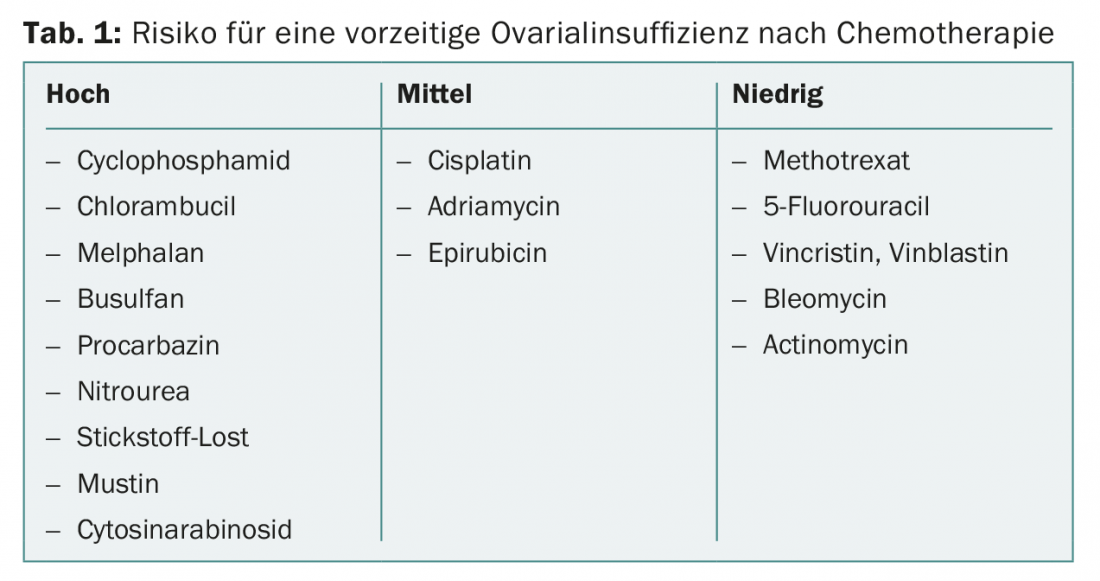
Treatments that have at least an 80% probability of causing persistent amenorrhea or azoospermia are considered severely gonadotoxic. These include, for example, total body irradiation as part of a stem cell transplant. In women over 40 years of age, menopause can be expected to occur within one year in 40-100% after chemotherapy with cyclophosphamide [7].
Therapies are considered moderately gonadotoxic, leading to amenorrhea in 40-60% of cases. These include, for example, adjuvant chemotherapy for breast cancer in women between the ages of 30 and 39, or second-line chemotherapy for Hodgkin’s disease .
For example, first-line chemotherapy according to the ABVD regimen in Hodgkin’s disease M. exhibits low gonadotoxicity. 45% of women with a median age of 26 years and 53% of men with a median age of 28 years achieved pregnancy after ABVD chemotherapy [8].
The number of primordial follicles present at birth decreases continuously in the female organism until menopause. This process is accelerated by cytotoxic therapies, resulting in earlier loss of ovarian function. Long-term survivors of childhood malignancy enter menopause significantly earlier than their sisters and therefore should not fulfill an existing desire to have children too late.
The likelihood of complete loss of ovarian function increases with age. Women over 40 years of age have a smaller oocyte reserve, so permanent loss of function must be expected in them even with radiation therapy with doses between 5 and 6 Gy. Younger women, on the other hand, may be able to tolerate up to 20 Gy. Radiation therapy to the pelvis in childhood or adolescence may result in reduced uterine volume, reduced endometrial thickness, or loss of uterine muscle elasticity.
Acute ovarian failure occurred in 215 (6.3%) of 3390 patients in the Childhood Cancer Survivor Study (CCSS) in the United States [9]. Among the 5149 female survivors aged 15-44 years, the pregnancy rate was 19% lower compared with unaffected siblings (RR 0.81; 95% CI 0.73-0.90).
Irregular cycles often indicate early onset of menopause. Decreased AMH (anti-Müllerian hormone) or inhibin B as well as increased FSH on the third day of the cycle and the lack of sonographic evidence of maturing follicles indicate a disturbance of ovarian function and are suitable as diagnostic criteria.
Male fertility after tumor therapy
In males, temporary oligospermia may occur at radiation doses as low as 0.1 Gy. Higher doses often result in complete interruption of sperm production. However, a return of fertility was sometimes recorded after years. Radiation doses above 6 Gy often cause permanent infertility. Data on the level of radiation doses associated with loss of fertility vary considerably in the literature [10].
30% of childhood tumor therapies result in gonadotoxic effects in boys with permanent infertility [11]. The extent and duration of damage depend on the cytostatic drug administered and the total dose. In particular, alkylants such as busulfan, cisplatin, cyclophosphamide, ifosfamide, and procarbazine are known to cause persistent infertility [12]. Most chemotherapies in children consist of combinations of several cytostatic agents, and synergistic effects can lead to infertility even at lower doses of the individual agents.
In addition to pathological spermiograms, low inhibin B and elevated FSH testify to the extent of impaired male fertility.
Fertility Protection
If gonadotoxic therapies are required in patients of fertile age, nowadays a detailed explanation of the options of fertility-preserving measures should be provided.
Therefore, reproductive medicine measures with cryopreservation of egg and sperm cells are to be discussed in the context of the therapy concept. While this is unproblematic with sperm, there are some critical issues with cryopreservation of fertilized embryos after in vitro fertilization: Hormonal stimulation of the ovary to obtain oocytes not only delays chemotherapy, but may also potentially stimulate tumor growth in hormone receptor-positive tumors.
If tumor treatment has resulted in azoospermia, sperm can also be surgically extracted from the testicular tissue using testicular sperm extraction (TESE).
Fertility preservation measures in women, on the other hand, are much more complicated and in some cases are only at the experimental stage.
The benefit of protecting the ovary by “immobilizing” the pituitary-ovarian axis during chemotherapy using LHRH agonists is controversial [13].
Ovarian stimulation treatment to obtain oocytes and cryopreservation of fertilized oocytes are established routine procedures in the context of in vitro fertilization. In specialized reproductive medicine centers, cryopreservation of unfertilized eggs is also performed. Ovarian stimulation to obtain oocytes can be performed in postpubertal women up to approximately 40 years of age. The chance of pregnancy after cryopreservation of fertilized oocytes before cytotoxic therapy is estimated to be 40% for patients 18-25 years of age, approximately 35% for patients 26-30 years of age, approximately 30% for patients 31-35 years of age, and approximately 25% for patients 36-40 years of age; these figures are cumulative pregnancy rates after multiple thaw cycles [14].
In contrast, laparoscopic harvesting and cryopreservation of ovarian tissue is still an experimental technique for fertility preservation. Young women with a high ovarian reserve are particularly suitable for this. However, when transplanting ovarian tissue, the potential risk of malignant cell transmission must also be considered [15].
For therapeutic pelvic irradiation, surgical transposition of the ovaries (oophoropexy) is an option to remove the ovarian tissue from the immediate radiation field.
Pregnancy rates after tumor diagnosis
There is often uncertainty about the necessary time interval between completion of chemotherapy and the onset of pregnancy. An interval of two years after completion of tumor therapy is often advised, especially since the risk of recurrence is greatest shortly after tumor therapy in aggressive tumors.
Among former pediatric oncology patients with an average age of about 24 years, the childbearing rate was 77% compared with 90% in the age-matched general population. In this context, the persons concerned mainly expressed fears that the child could also develop cancer or that their own disease could break out again [16].
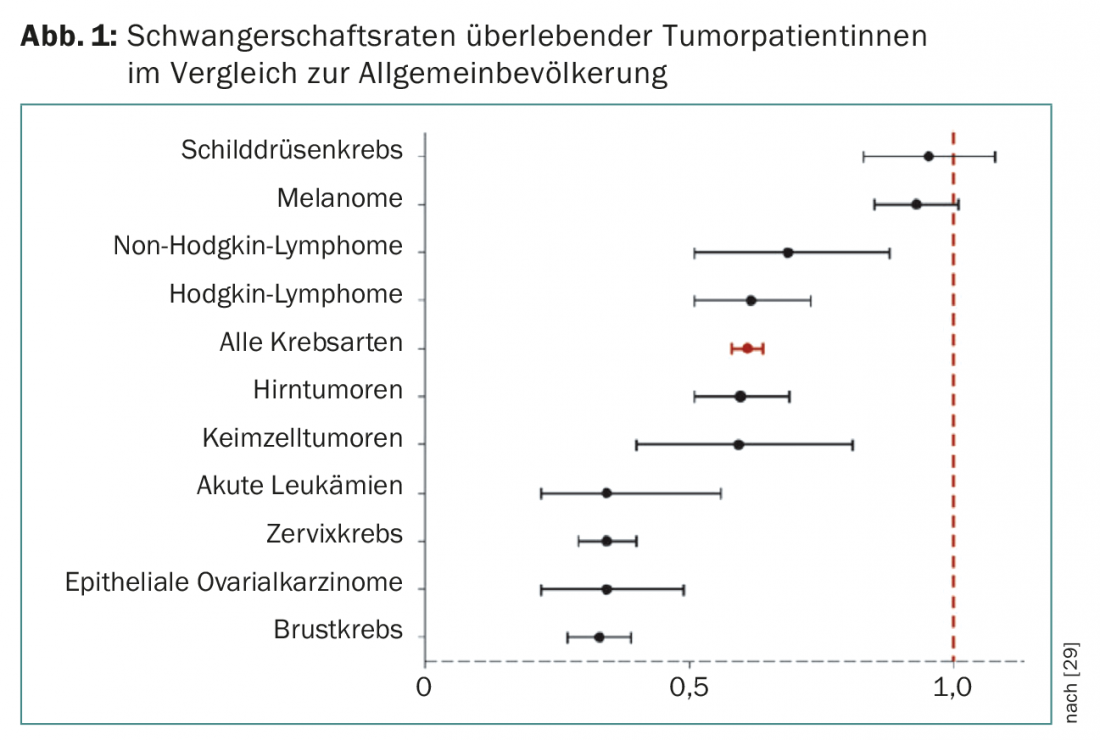
Pregnancy rates of successfully treated tumor patients are significantly lower than those reported in the comparable general population, depending on tumor type (Fig. 1) [17].
Tumor prognosis after pregnancy
Depending on the type of tumor, those affected are sometimes also concerned about the extent to which pregnancy could worsen the prognosis of the underlying maternal disease. Obviously, however, a later pregnancy has no negative influence on the prognosis. This is also true for hormone-dependent tumors such as breast carcinoma [18]. In a recent study, no difference in disease-free survival was observed in 333 patients with estrogen receptor-positive breast carcinoma and subsequent pregnancy at 7.2 years of follow-up compared with a control population without gravidity [19]. A meta-analysis of 14 studies on the prognosis of breast carcinoma found a 41% lower mortality rate after the onset of pregnancy compared with breast cancer patients without gravidity [20]. However, this may also be due to the fact that patients with a good prognosis after tumor therapy are more likely to have the courage to realize their desire to have a child.
Effects of oncologic therapy on offspring.
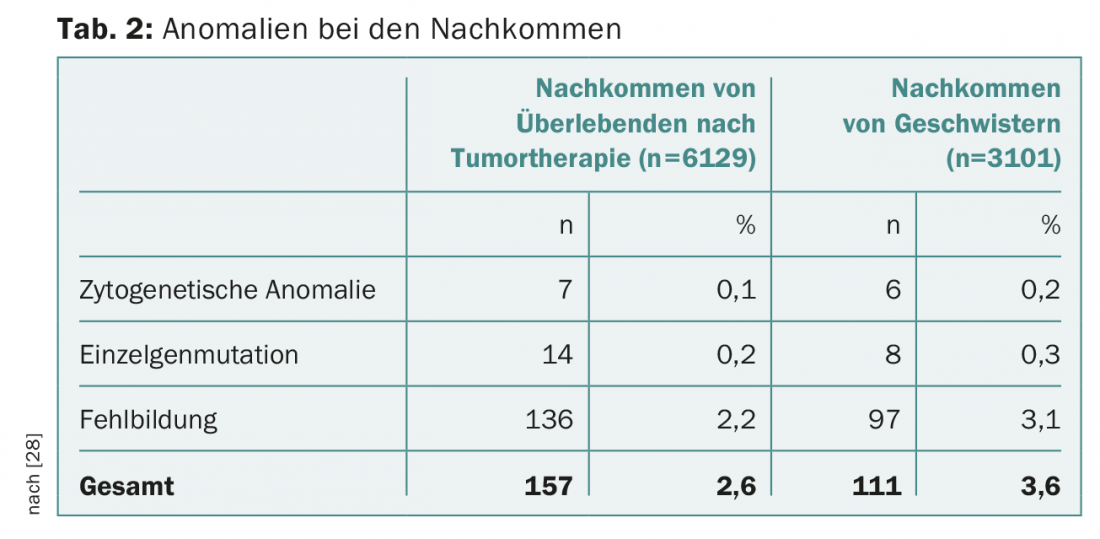
After successful tumor therapy, expectant parents are often burdened by questions about possible damage to their offspring. The risk of malformation in children is not increased after parental chemotherapy, according to current knowledge. Follow-up of over 4000 pregnancies after maternal chemotherapy showed no increase in fetal malformation rate [21]. The U.S. National Cancer Institute database includes more than 10,000 patients with childhood or adolescent malignancy treated between 1970 and 1986. Birth weight below the tenth percentile was registered significantly more often, 18.2% vs. 7.8% (OR 4.0; 95% CI 1.6-9.8; p=0.003), especially after uterine radiotherapy over 5 Gy [22]. However, there was no increase in congenital organ abnormalities, cytogenetic syndromes, or genetic defects in the offspring (Table 2).
A retrospective cohort study using data from cancer and birth registries in four U.S. regions analyzed the offspring of 1898 tumor patients with an initial diagnosis before age 20 (period: 1973-2000) compared with an unaffected control group (n=14 278). There was a significant increase in preterm births (RR 1.54; 95% CI 1.30-1.83) and offspring with a birth weight below 2500 g (RR 1.31; 95% CI 1.10-1.57). However, again, no increase in malformation rates, intrauterine fetal death, or chromosomal aberrations was observed in the offspring [23]. A corresponding evaluation of pregnancies of male tumor survivors (n=470) showed no increase in preterm births, growth retardation, malformation rates, or chromosomal aberrations [24].
A recent review considers reproductive outcome after successful therapy for childhood and adolescent leukemias and lymphomas, including 18 relevant studies [3]: No increase in spontaneous abortions, maternal diabetes or anemia, stillbirths, fetal malformations, or tumors in offspring was found among long-term survivors. However, the rate of live births was lower compared to an unaffected control population, while preterm birth and low birth weight were more common.
Tumor therapy during pregnancy
When a tumor is first diagnosed during pregnancy, there are therapeutic procedures that are quite tolerable for both mother and fetus. Maternal and fetal well-being must be weighed when choosing treatment.
Breast carcinoma, cervical carcinoma, hematologic malignancies, and melanoma account for 70% of all tumor diagnoses in pregnancy [25].
Whether systemic therapy is started in pregnancy depends on tumor stage, gestational age, tumor type, expected benefits, and risks to be feared. Because cytostatic drugs can impair organogenesis, there is a 10-20% risk of malformation with chemotherapy in the first trimester. In contrast, the miscarriage rate with application of cytostatic drugs in the second and third trimesters corresponds to the general baseline risk of 3-5% [26]. The greatest experience in pregnancy is available for breast carcinoma: Therapy regimens with anthracyclines such as FEC (5-fluorouracil, epirubicin, cyclophosphamide) or FAC (5-fluorouracil, doxorubicin, cyclophosphamide) have performed well [27]. Taxanes and platinum compounds can also be used in pregnancy.
Immunotherapies currently lack sufficient evidence in pregnancy. Hormone therapy should be avoided during pregnancy. In terms of pregnancy outcome, a moderate increase in preterm birth and growth restriction is emerging.
Literature:
- Federal Statistical Office (FSO): Swiss Cancer Report 2015. Status and developments. Neuchâtel 2016.
- Eibye S, Kjaer SK, Mellemkjaer L: Incidence of pregnancy-associated cancer in Denmark 1997-2006. Obstet Gynecol 2013; 122: 608-617.
- Shliakhtsitsava K, et al: Pregnancy and child health outcomes in pediatric and young adult leukemia and lymphoma survivors: a systematic review. Leuk Lymphoma 2018; 59(2): 381-397.
- Kort JD, et al: Fertility issues in cancer survivorship. CA Cancer J Clin 2014 Mar-Apr; 64(2): 118-134.
- Lambertini M, et al: Cancer and fertility preservation: international recommendations from an expert meeting. BMC Med 2016; 14: 1.
- Balachandren N, Davies M: Fertility, ovarian reserve and cancer. Maturitas 2017; 105: 64-68.
- Gadducci A, Cosio S, Genazzani AR: Ovarian function and cildbearing issues in breast cancer survivors. Gynecol Endocrinol 2007; 23(11): 625-631.
- Boltežar L, Pintarić K, Jezeršek Novaković B: Fertility in young patients following treatment for Hodgkin’s lymphoma: a single center survey. J Assist Reprod Genet 2016; 33(3): 325-333.
- Metzger ML, et al: Female reproductive health after childhood, adolescent, and young adult cancers: guidelines for the assessment and management of female reproductive complications. J Clin Oncol 2013; 31(9): 1239-1247.
- Bruhn C: Pregnancy after or during cancer. Dtsch Med Wochenschr 2014; 139: 1146-1147.
- Green DM, et al: Fertility of male survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. J Clin Oncol 2010; 28(2): 332-339.
- Ginsberg JP: Educational paper: the effect of cancer therapy on fertility, the assessment of fertility and fertility preservation options for pediatric patients. Eur J Pediatr 2011; 170(6): 703-708.
- Senra JC, et al: Gonadotropin-releasing hormone agonists for ovarian protection during cancer chemotherapy: systematic review and meta-analysis. Ultrasound Obstet Gynecol 2017 Oct 21. DOI: 10.1002/uog.18934 [Epub ahead of print].
- Wunder C, et al: Swiss recommendations on fertility preservation for patients of fertile age before cytotoxic therapies. Schweiz Med Forum 2012; 37: 708-709.
- American Society for Reproductive Medicine, Practice Committee: Ovarian tissue cryopreservation: a committee opinion. Fertil Steril 2014; 101(5): 1237-1243.
- Reinmuth S, et al: Having children after surviving cancer in childhood or adolescence – results of a Berlin survey. Clin Padiatr 2008; 220: 159-165.
- Stensheim H, et al: Pregnancy after adolescent and adult cancer: a population-based matched cohort study. Int J Cancer 2011; 129: 1225-1236.
- Partridge AH, Ruddy KJ: Fertility and adjuvant treatment in young women with breast cancer. Breast 2007; 16(Suppl 2): S175-181.
- Lambertini M, et al: Long-term Safety of Pregnancy Following Breast Cancer According to Estrogen Receptor Status. J Natl Cancer Inst 2017 Oct 26. DOI: 10.1093/jnci/djx206 [Epub ahead of print].
- Azim HA Jr, et al: Safety of pregnancy following breast cancer diagnosis: a meta-analysis of 14 studies. Eur J Cancer 2011; 47(1): 74-83.
- Hawkins MM: Pregnancy outcome and offspring after childhood cancer. BMJ 1994; 309(6961): 1034.
- Green DM, et al: Ovarian failure and reproductive outcomes after childhood cancer treatment: results from the Childhood Cancer Survivor Study. J Clin Oncol 2009; 27(14): 2374-2381.
- Mueller BA, et al: Pregnancy outcomes in female childhood and adolescent cancer survivors: a linked cancer-birth registry analysis. Arch Pediatr Adolesc Med 2009; 163(10): 879-886.
- Chow EJ, et al: Reproductive outcomes in male childhood cancer survivors: a linked cancer-birth registry analysis. Arch Pediatr Adolesc Med 2009; 163(10): 887-894.
- Boere I, et al: Cancer in pregnancy: safety and efficacy of systemic therapies. Curr Opin Oncol 2017; 29(5): 328-334.
- Ngu SF, Ngan HY: Chemotherapy in pregnancy. Best Pract Res Clin Obstet Gynaecol 2016; 33: 86-101.
- Shachar SS, et al: Multidisciplinary Management of Breast Cancer During Pregnancy. Oncologist 2017; 22(3): 324-334.
- Green DM, et al: Fertility of female survivors of childhood cancer: a report from the childhood cancer survivor study. J Clin Oncol 2009; 27(16): 2677-2685.
- Peccatori FA, et al: Cancer, pregnancy and fertility: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013; 24(Suppl 6): vi160-170.
InFo ONCOLOGY & HEMATOLOGY 2017; 5(6): 27-30.